Introduction to Biological Macromolecules Study Guide
Introduction
Living organisms require nutrients for the functioning and development of their organ systems. The nutrients are synthesized by all life forms at the cellular level. To carry out the organ system’s development and nourishment, these biological macromolecules or Bio-Macromolecules act as “building blocks.” They facilitate optimum development, growth, and functioning in practically all life forms.
The term biological macromolecules were first coined in the 1920s by a Molecular Scientist named Herman Staudinger. He was awarded the Nobel Prize for his contribution to molecular sciences. Based on his observations, he established that these biological macromolecules result from the linkage of two kinds of bio-molecules, monomers and polymers.
Monomers are the smaller sub-units that combine to form a polymer. Repeating units of these polymers combine to form a polymer, i.e., a larger molecule. These larger molecules then combine to form a chain. These smaller units, called monomers, are combined to make Polymers. These organic bio-polymers can further be classified into four broad categories of macromolecules.
What are macromolecules?
Biological macromolecules are very large molecules formed due to the polymerization of smaller molecules called monomers.
Types of biomolecules
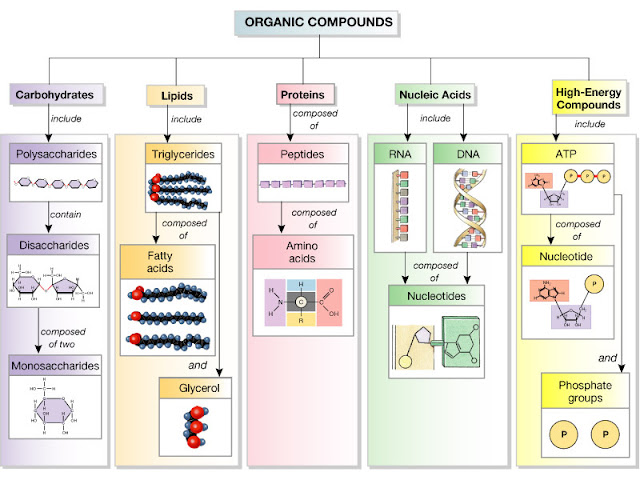
Macromolecules are broadly classified as:
- Carbohydrates
- Lipids
- Proteins
- Nucleic Acids
1. Carbohydrates: Carbohydrates are polymers of carbon, hydrogen, and oxygen. They are further classified as monosaccharides, disaccharides, and polysaccharides. Carbohydrates are found in starch, fruits, vegetables, milk, and sugars.
2. Nucleic Acids: The nucleic acids include DNA and RNA, which are the polymers of nucleotides. Nucleotides are further comprised of a pentose group, a phosphate group, and a nitrogenous base group. All hereditary or genetic information is stored in the DNA. DNA is synthesized into RNA and proteins.
3. Proteins: Proteins are the polymers of amino acids, including the carboxylic and the amino group. Lipids and carbohydrates are non-existent without proteins because the enzymes used for their synthesis are proteins themselves.
4. Lipids: Lipids are a hydrophobic set of macromolecules because they do not dissolve in water. Lipids comprise triglycerides, carotenoids, phospholipids, and steroids. They help in the formation of the cell membrane, formation of hormones, and store fuel.
Chemical bond, metallic bond, and covalent bond types:
A chemical bond is the formation of a chemical bond between two or more atoms, molecules, or ions, which results in a chemical compound. The chemical bonds ensure that the atoms remain together in the resulting compound. The chemical bonds formed change in strength and properties. The four primary types of chemical bonds are Ionic bonds, covalent bonds, hydrogen bonds, and polar bonds.
A metallic bond is a chemical bond formed between atoms in a metallic element. Metallic bonds are observed in pure metals, alloys, and some metalloids. Metallic bonding imparts properties to metals, which are of commercial significance. These properties include electrical conductivity, thermal conductivity, malleability and ductility, metallic luster, high melting and boiling point.
A covalent bond is formed by the equal sharing of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair. The covalent bonds are also called molecular bonds. Covalent bonds’ key properties include: they are very powerful chemical bonds; they rarely break spontaneously after formation; most compounds having covalent bonds display relatively low melting points and boiling points.
Bond development of monomers
Dehydration Synthesis:
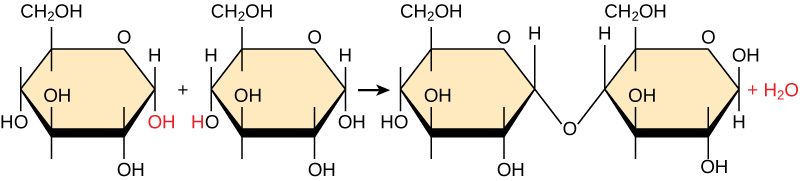
Most macromolecules are made from single subunits called Monomers. The monomers combine using covalent bonds to form Polymers – larger molecules, releasing water molecules as byproducts. This reaction process involving the release or loss of water is called dehydration synthesis.
In a dehydration synthesis reaction (figure above), the hydrogen of one monomer combines with the hydroxyl group of another monomer, releasing a water molecule. Simultaneously, the monomers share electrons and form covalent bonds. As additional monomers join, the chain of repeating monomers forms a polymer.
Hydrolysis:
Hydrolysis is the process of Polymers breaking down into monomers. Insertion of a water molecule across the bond triggers a chemical reaction, breaking the covalent bond with this water molecule. During these reactions, the polymer breaks into two parts: one part gains a hydrogen atom (H+), and the other gains a hydroxyl molecule (OH–) from a split water molecule.
- Specific enzymes catalyze dehydration and hydrolysis reactions
- Dehydration reactions involve the formation of new bonds, which requires energy
- Hydrolysis reactions break bonds, releasing energy in the process
Monomers and Polymers:
Macromolecules are essentially polymers. The long chains of molecular sub-units are called Monomers. Carbohydrates, proteins, and nucleic acids occur as long polymers. Due to their polymeric nature and large size, they are known as macromolecules.
Monomers are small molecules that bond together to form more complex structures such as polymers. There are four main monomer types: sugars, amino acids, fatty acids, and nucleotides. Glucose, vinyl chloride, amino acids, and ethylene are monomers.
Conclusion:
- Macromolecules are very large biological molecules such as carbohydrates, proteins, lipids, and nucleic acids.
- An individual unit of the macromolecule is known as a monomer.
- Macromolecules are long polymer chains of many molecules.
- Macromolecules are formed by the dehydration reaction and can be broken down by the hydration reaction.
FAQs:
1. What is a biological macromolecule? What are biological molecules?
Biological macromolecules are very large molecules formed due to the polymerization of smaller molecules called monomers.
2. What are the four major biological macromolecules?
The four major biological macromolecules are Carbohydrates, Lipids, Proteins, and Nucleic acids.
3. What are biological macromolecules called?
Biological macromolecules are also called proteins, carbohydrates, nucleic acids, and lipids based on their different composition.
4. Why are biological macromolecules important in everyday life?
The four types of macromolecules, proteins, carbohydrates, nucleic acids, and lipids, play important roles in the life of a cell. Their role in everyday life includes:
- Providing structural support
- Being a source of stored fuel
- storing and retrieving genetic information
- In hastening biochemical reactions
5. What are the three biological molecules that make up the DNA?
The three biological molecules that make up DNA are the phosphate group, a sugar group, and four nitrogen bases.
6. Why are biological macromolecules considered organic?
Biological macromolecules are considered organic as they contain carbon.
We hope you enjoyed studying this lesson and learned something cool about the Introduction to Biological Macromolecule! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
Sources:
- Properties of Biological Macromolecules. https://biologydictionary.net/ap-biology/1-4-properties-of-biological-macromolecules/. Accessed 16 Dec, 2021
- Properties of Polymers. https://courses.lumenlearning.com/boundless-chemistry/chapter/properties-of-polymers/. Accessed 16 Dec, 2021
- Molecular Structure and Function. https://www.ncbi.nlm.nih.gov/books/NBK217812/. Accessed 16 Dec, 2021