Enzyme Catalysis Study Guide
Introduction:
Enzymes as catalysts
Enzymes are biological catalysts; they are substances that speed up a chemical reaction—without being a reactant. Therefore, as catalysts, enzymes are only required in very low concentrations as they are not consumed during the reaction.
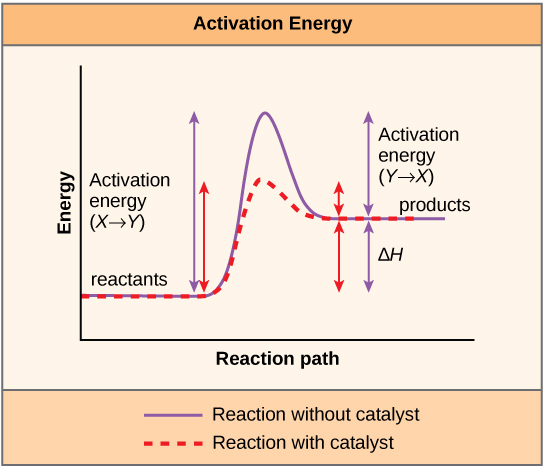
A chemical reaction is the conversion of substrate molecules into product molecules. The substrate must first be converted to a higher energy state, called the transition state, for this reaction to occur. The energy necessary to reach the transition state is called the activation energy. Enzymes act by reducing the activation energy, thereby increasing the speed of the reaction.
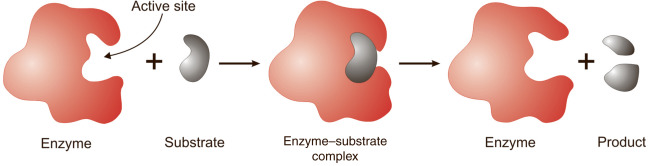
Most enzymes are proteins made up of chains of amino acids that bond with one another. The amino acid sequence determines the specific folding patterns of the protein’s structure: primary, secondary, tertiary, and quaternary. Amino acid-based enzymes are globular proteins. Enzyme catalysis begins when the enzyme binds its substrate to form an enzyme-substrate complex. The substrate binds to a specific region of the enzyme, called the active site. While attached to the active site, the substrate is converted into the product of the reaction, which is then released from the enzyme.
Substrate Specificty
Substrate specificity is one of the most vital distinctive features of enzymes. In the active site of the enzymes, there is a unique combination of amino acid residues called side chains, or R groups, each characterized by distinct properties. Residues can be weakly acidic or basic, large or small, hydrophilic or hydrophobic, positively or negatively charged, or neutral. These amino acids’ shape and charge properties in the active site ensure it binds to a single type of substrate molecule. This, in turn, helps the enzyme demonstrate considerable specificity in its catalytic activity.
How do enzymes work?
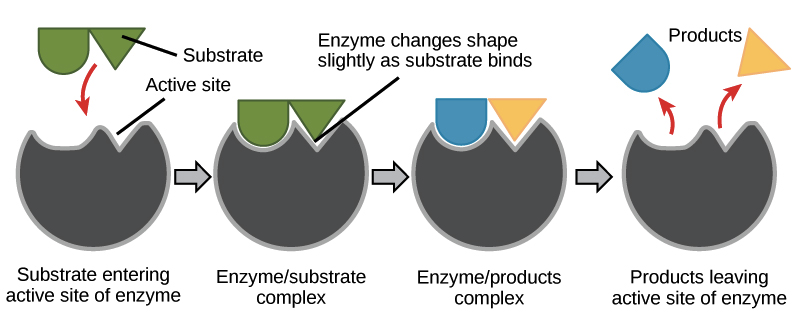
Enzyme- substrate binding is described by the induced fit model in which the enzyme molecule changes its conformation slightly to accommodate the binding of the substrate. After the enzyme and substrate have bound to each other, the enzyme lowers the reaction’s activation energy by altering the reaction’s transition state, allowing it to happen more quickly.
These are a few ways enzymes can catalyze reactions:
- They help position the substrates together in an optimal orientation.
- It establishes an ideal environment within the active site for the reaction to proceed.
- Breaking of bonds to achieve the transition state is enhanced by contorting substrate molecules
- Involvement of active-site residues that form covalent bonds with substrate molecules in the formation and stabilization of the transition state
It is to be noted that the enzyme is not consumed in the reactions they catalyze; it just releases the product and returns to its original state to participate in further catalysis.
Cofactors and coenzymes
Bound to some enzymes is an additional chemical component called a cofactor and a coenzyme. For the enzyme to work, it must be activated by binding these inorganic chemicals and organic compounds, respectively. Binding to these molecules promotes optimal conformation as they play a central role in the catalytic process. Cofactors are inorganic ions such as iron, zinc, and magnesium, and the most common sources of coenzymes are dietary vitamins. For example, Vitamin C is a coenzyme for multiple enzymes that build up our collagen tissue. Most vitamins are essential in nutrition because they act as coenzymes or raw materials from which coenzymes are made.
Enzyme regulation
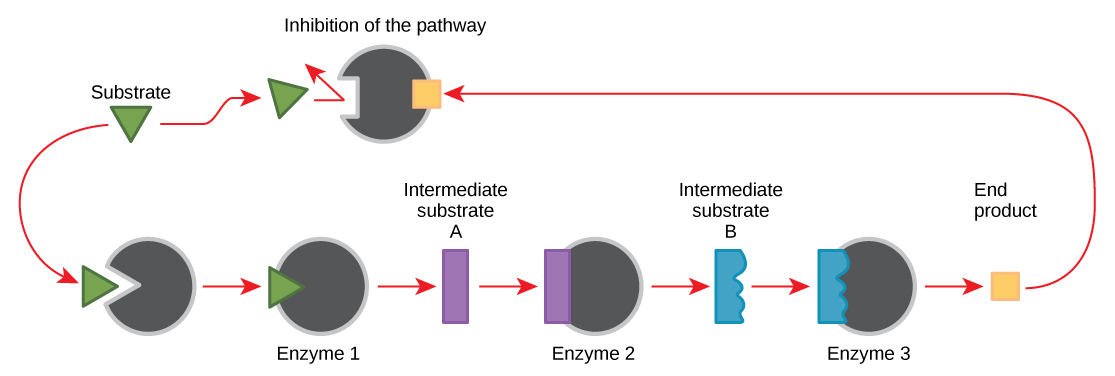
The catalytic activity of an enzyme can be regulated. One common type of enzyme regulation is feedback inhibition, in which the product of a metabolic pathway inhibits the action of an enzyme involved in its synthesis. It is an essential regulatory mechanism in cells, especially in the production of both amino acids and nucleotides.
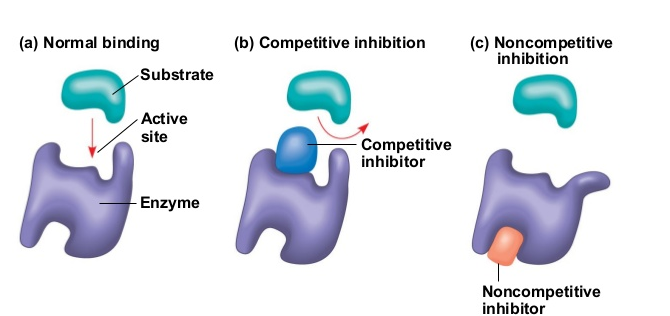
Inhibitors are molecules that reduce the rate of an enzyme-catalyzed reaction by binding to an enzyme, some temporary or others permanently, such as poisons.
Competitive Inhibition- occurs when molecules very similar to the substrate molecules bind to the active site and prevent binding of the actual substrate. Since the structurally related inhibitor molecule competes with the substrate for active site binding, it is called competitive inhibition. When the inhibitor occupies the active site, it forms an enzyme-inhibitor complex, and the enzyme cannot react until the inhibitor dissociates. High substrate concentrations can fully overcome this type of inhibition as the substrate will ultimately occupy all the active sites.
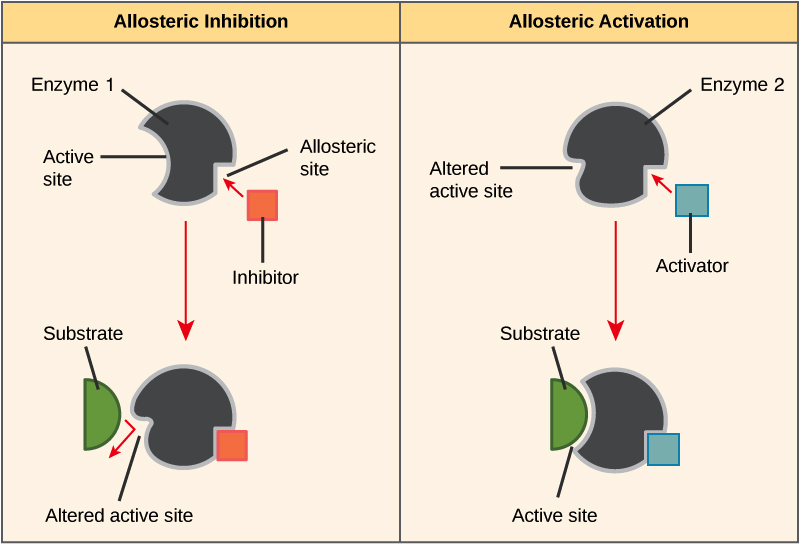
Non-competitive Inhibition- occurs when an inhibitor binds to the enzyme at a location other than the active site, called the allosteric site. This binding induces changes in the conformation of the protein, which in turn alters the shape of the active site to prevent binding by the substrate and thus inhibits the enzyme’s catalytic activity. This type of non-competitive inhibition is called allosteric inhibition.
When a reaction product of an anabolic or catabolic reaction pathway serves as an allosteric inhibitor on an earlier pathway enzyme, this inhibition of an enzyme also becomes a form of negative feedback. There are allosteric activators as well as allosteric inhibitors. An allosteric activator causes a conformational change that increases the affinity of an enzyme for the substrate, thus increasing the rate of reaction.
Conclusion:
- Enzymes are proteins that help speed up the chemical reactions in our bodies. Enzymatic reactions thus play an important role in controlling most life processes.
- They are also used in industrial processes, such as fermented products, detergents, baking, brewing, and pharmaceuticals.
FAQs:
1. Why do all enzymatic reactions need activation energy?
Activation energy is defined as the energy required to start a reaction. Activation energy is needed to disrupt the stable electron configuration of a substrate. The lower the activation energy for a reaction, the faster is its rate.
2. What are enzymes?
Enzymes are proteins that act as biological catalysts and help speed up metabolism, or the chemical reactions in our bodies.
3. How does a competitive inhibitor slow enzyme catalysis?
Competitive inhibitors compete with the substrate for the enzyme’s active site.
4. What is enzyme catalysis?
Enzyme catalysis is the increase in the rate of a process by a biological molecule, an “enzyme”. Enzymes can catalyze reactions through a variety of mechanisms.
5. In what way does the binding of a substrate by an enzyme enhance catalysis?When an enzyme binds its substrate, it forms an enzyme-substrate complex. The binding energy of the enzyme-substrate interaction lowers the activation energy of the reaction and promotes its rapid progression.
6. What property of a metal ion makes it a useful cofactor in enzyme-mediated catalysis?
Metal ion cofactors play various roles to enhance the catalytic efficiency of enzymes. These include:
- Functioning as a Lewis acid to stabilize the formation of the leaving group
- Functioning as an electrostatic catalyst
- Facilitation of substrate binding
- Gathering/template effects
We hope you enjoyed studying this lesson and learned something cool about Enzyme Catalysis! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun 😎
Sources:
- “Enzyme Substrate Complex – an Overview | ScienceDirect Topics.” Www.sciencedirect.com, www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/enzyme-substrate-complex. Accessed 30 Dec, 2021.
- “Enzymes and the Active Site.” Khan Academy, 2015, www.khanacademy.org/science/ap-biology/cellular-energetics/enzyme-structure-and-catalysis/a/enzymes-and-the-active-site. Accessed 30 Dec, 2021.
- “Enzyme Regulation (Article).” Khan Academy, www.khanacademy.org/science/ap-biology/cellular-energetics/environmental-impacts-on-enzyme-function/a/enzyme-regulation. Accessed 30 Dec, 2021.