CBSE Class 10 Science Chapter 4 Revision Notes
Chapter 4: Carbon and its Compounds Revision Notes
- Carbon is a very adaptable element.
- Carbon is found in .02 percent in the form of minerals and .03 percent in the form of all biological structures.
Covalent Bond in Carbon
- Carbon has an atomic number of 6 and an electrical configuration of 2, 4.
- It takes four extra electrons in its valence shell to achieve a noble gas structure.
- It is tough for a carbon atom to obtain or lose electrons since it is difficult to hold additional electrons and removing four electrons requires a lot of energy.
- The noble gas configuration is achieved by sharing valence electrons with other elements.
- Covalent bonding refers to the mutual sharing of electrons between atoms in order to achieve a stable noble gas structure.
- Valence electrons are shared by atoms of other elements such as hydrogen, oxygen, nitrogen, and chlorine.
Covalent bonds are classified into three categories based on the number of electron pairs shared:
- Single Covalent Bond: A single covalent bond is created when two electrons, or one pair, are shared.
Hydrogen, chlorine, and methane are three examples.
- Double covalent bond: A double bond is produced when two pairs of electrons share four electrons.
A good example is the molecule of oxygen and the molecule of carbon dioxide.
-
Triple Covalent Bond: A triple covalent bond is established when six electrons, three pairs of electrons, are shared.
Nitrogen (C2H2) is an example of a nutrient.
Covalently bound molecules have low melting and boiling points.
- Because no charged particles are generated, these molecules are poor conductors of electricity.
Carbon has two crucial qualities that allow it to build a huge variety of compounds.
Catenation
- The ability of a carbon atom to make bonds with other carbon atoms is known as catenation.
- Silicon, like carbon, creates silanes, which are silicon compounds containing up to seven or eight hydrogen atoms.
Tetra Valency
- A carbon atom with a valency of four may bind with atoms of oxygen, hydrogen, nitrogen, sulphur, chlorine, and other elements.
- Carbon is referred regarded as tetravalent because it requires four electrons.
- Because of the smaller size of the carbon atom, the nucleus can tightly hold the shared pair of electrons, making carbon compounds particularly stable in general.
Hydrocarbons
- Compounds consisting mostly of hydrogen and carbon atoms.
SATURATED AND UNSATURATED CARBON COMPOUNDS
Saturated Compounds
- Saturated compounds are hydrocarbons with single bonds between hydrogen and carbon. Alkanes are another name for them.
Unsaturated Compounds
- They are hydrocarbons with double or triple bonds between hydrogen and carbon. Alkenes are hydrocarbons with double covalent bonds, whereas alkynes have triple covalent bonds.
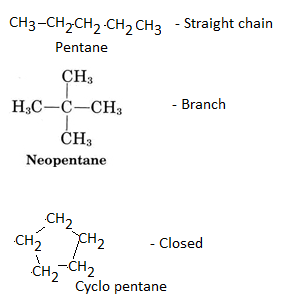
Carbon compounds are also found in cyclic form, in addition to branching forms.
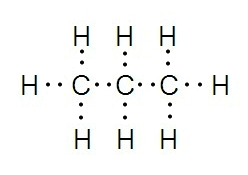
Lewis structures (electron dot structures) are diagrams that depict the bonding between atoms in a molecule and any lone pairs of electrons that may be present in the molecule.
The following are the steps to drawing a Lewis structure:
-
Determine how many valence (outer shell) electrons each atom in the molecule possesses.
-
In a molecule with many atom types, place the most metallic or least electronegative atom in the middle. Remember that when an atom advances away from fluorine on the periodic table, its electro-negativity diminishes.
-
Assemble the electrons such that each atom provides one electron to each atom’s single bond.
-
Are the octets complete? Count the electrons around each atom. If that’s the case, your Lewis dot structure is finished.
-
If the octets aren’t full and extra electrons are needed, shift one electron per bond per atom to form a new bond. There will be open octets in some configurations (for example, the B in BF3), or atoms with 10 electrons (example: the S of SF5)
-
Continue with steps 4 and 5 until all octets are filled.
-
Redraw the dots such that, wherever feasible, electrons on each atom are in pairs.
NOMENCLATURE
- The system of assigning names to chemical substances is known as nomenclature.
Functional Groups and Heteroatoms
- One or more hydrogen atoms can be substituted with other atoms in a hydrocarbon chain based on their valencies.
- A hetero atom is the element that substitutes hydrogen.
- Functional groups are made up of hetero atoms and the groups that contain them, which provide the molecule chemical characteristics.
Homologous Series
- It’s a collection of molecules in which one functional group replaces hydrogen in a carbon chain.
- Each subsequent component differs by one -CH2 unit and 14 mass units.
- The functional group imparts chemical traits, therefore all members have comparable chemical properties. The members, however, have various morphological characteristics.
- Due to differences in molecular mass, the physical characteristics of homologous series members differ.
- With increasing molecular mass, the melting and boiling points rise.
Naming a carbon compound
-
Determine how many carbon atoms are in the chemical.
-
A prefix or suffix is used to designate a functional group.
Functional Group Suffix Prefix
Alkene- ene
Alkyn- yne
Alcohol- ol
Aldehyde- al
Ketone- one
Carboxylic acid- oic acid
chlorine -chloro
- If a suffix is added, the last ‘e’ from the name is eliminated, for example, methane-e (methan + ol).
CHEMICAL CHARACTERISTICS
Reactions of Combustion
- Combustion is the process of converting carbon or carbon-containing substances into carbon dioxide, heat, and light in the presence of air or oxygen.
Characteristics of the Flame
- Unsaturated hydrocarbons produce a smoky flame, but saturated hydrocarbons provide a clear flame.
- Even saturated hydrocarbons produce a hazy blaze when oxygen is scarce.
Addition
- Addition reactions occur when two molecules react to generate a single product that contains all of the combining components’ atoms.
- The addition process is exemplified by the hydrogenation reaction.
- Hydrogen is added to a double or triple bond in the presence of a catalyst such as nickel, palladium, or platinum in this reaction.
Substitution
- A substitution reaction occurs when an atom or group of atoms in a molecule is replaced or substituted by another atom or group of atoms.
- Hydrogen atoms are replaced by other elements in alkanes.
CH4+Cl2+Sunlight → CH3Cl+HCl
Ethanol
(i) Ethanol, C2H5OH is a colourless liquid having a pleasant smell.
(ii) It boils at 351 degrees Fahrenheit.
(iii) It is miscible in all amounts with water.
(iv) It is an electrical nonconductor (it does not contain ions)
(v) It has no effect on litmus.
Uses-
-
As an antifreeze in automobile radiators in cold countries.
-
In the production of paints, dyes, pharmaceuticals, soaps, and synthetic rubber as a solvent.
-
As a solvent for making iodine tincture.
What Effects Do Alcohols Have on People?
(i) When ethanol is combined with CH3OH and ingested, it results in severe poisoning and vision loss.
(ii) It induces addiction and harms the liver if used in large quantities.
(iii) Excessive ethanol drinking can result in death.
Ethanol Reactions with Sodium
When ethanol interacts with sodium, hydrogen gas and sodium ethoxide are produced. The acidic nature of ethanol is supported by this reaction.
2C2H5OH+2Na → 2C2H5ONa+H2
(↑)
Reaction of Elimination
- A sort of reaction in which two substituents are eliminated from a molecule is known as an elimination reaction.
- In the preparation of alkenes, these reactions are crucial.
Reaction to Dehydration
- At 443 K, ethanol combines with concentrated sulphuric acid to form ethylene.
- Because a water molecule is removed from the ethanol molecule during this process, it is called dehydration of ethanol.
CH3CH2OH → CH2=CH2+H2O
(The reaction occurs in the presence of Conc.H2SO4)
Ethanoic acid (ethanol) or acetic acid (acetic acid)
(i) Molecular formula: CH3COOH
(ii) It is water, alcohol, and ether soluble.
(iii) Because it freezes often during the winter in frigid climates, it is known as glacial acetic acid.
Esterification
- A sweet-smelling ester is generated when a carboxylic acid is refluxed with alcohol in the presence of a little amount of conc.H2SO4.
- Esterification is the process of forming an ester.
Saponification
- A sodium or potassium salt of long-chain carboxylic acids is referred to as soap (fatty acid).
- RCOONa is a general representation of the soap molecule, with R denoting the non-ionic hydrocarbon group and COONa+ denoting the ionic group.
- Hydrolysis of fat occurs when oil or fat of vegetable or animal origin is treated with a strong sodium or potassium hydroxide solution, resulting in soap and glycerol formation.
- Saponification is the term for the alkaline hydrolysis of oils and fats.
Source:
]]>