Acids and Bases Study Guide
Introduction
Lemonade on a hot summer day is the most refreshing! Have you ever wondered where the delicious sour taste comes from? Lemons get their sourness from citric acid, present in almost all citrus fruits. The word acid comes from the Latin word ‘acere,’ meaning ‘sour.’ There are thousands of other ways you interact with acids and bases in your daily life. They are an integral part of our lives. (Interestingly, even the above-mentioned soft drinks contain an acid known as carbonic acid.
What are Acids and Bases?
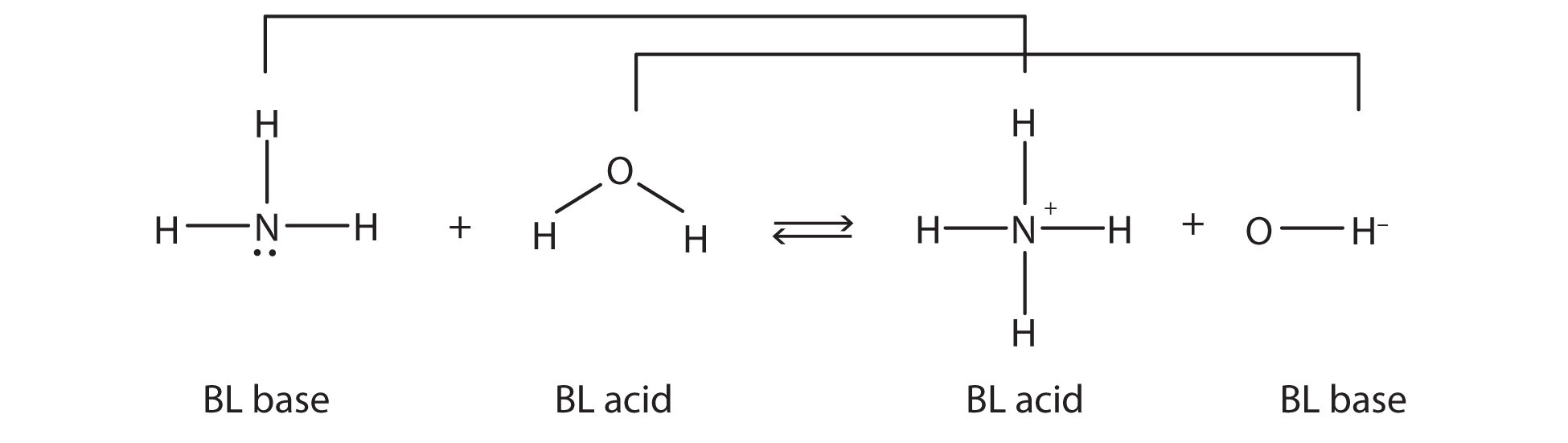
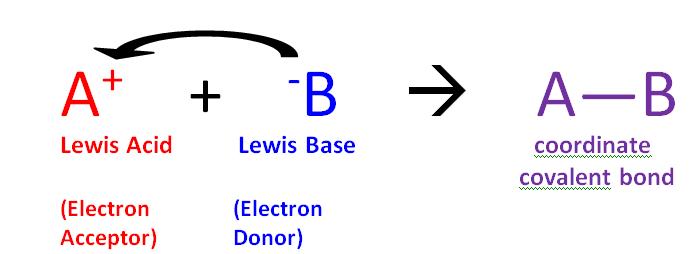
- Acids are defined as compounds capable of donating a proton (Brønsted-Lowry) or capable of forming a covalent bond with an electron pair (Lewis acid).
- Conversely, bases are defined as compounds capable of accepting a proton or donating an electron pair to form a covalent bond.
Characteristics of Acids and Bases
Characteristics of Acids
- Sourness: Acids, even diluted ones, have a sour taste
- They turn blue litmus paper red and phenolphthalein indicator colorless
- React with more reactive metals to release hydrogen in a single-displacement reactionZn(s) + H₂SO₄ (aq) —> ZnSO₄ (aq) + H₂ (g)
- Their aqueous solutions (acid in water) conduct electric current, with varying efficiencies
Characteristics of Bases
- Bitterness: Bases are bitter to taste
- They turn red litmus paper blue and phenolphthalein indicator pink
- They do not react with metals as acids do
- Their aqueous solutions conduct electricity as well
Bronsted-Lowry Acid examples:
- Hydrochloric acid (HCl)
- Nitric acid (HNO3)
- Hydrobromic acid (HBr)
- Water (H2O)
- Hydronium ion (H3O )
Lewis Acid examples:
- Copper (Cu2)
- Ion (Fe2 and Fe3)
- Hydrogen ion (H )
- All cations (Cu 2, Zn 2, Fe 2, Fe3 )
- An atom or ion or molecule with an incomplete octet of electrons: like AlF3 (Aluminium fluoride)
Everyday Examples of Acids
There are countless ways we interact with acids on a day-to-day basis. Examples of acids are:
- Citrus fruits have citric acid (C₆H₈O₇)
- Apples have malic acid (C₄H₆O₅)
- Milk releases lactic acid when digested (C₃H₆O₃)
- Butyric acid is present in butter (C₄H₈0₂)
- Older batteries often have sulphuric acid to help facilitate the storage of energy (H₂SO₄)
- Vinegar, often used in cooking, is diluted acetic acid with a few additions (CH₃COOH)
- Tea has not only tannic acid (C76H52O46) but also contains oxalic acid, also present in coffee and peppers (C2H2O4)
Everyday Examples of Bases
Just like acids, bases also play a key part in our lives. Examples of bases are:
- Toothpaste is slightly basic due to the presence of sodium fluoride. It helps neutralize the acidity in our mouth, further preventing bacterial build-up (the bacteria like an acidic environment).
- Soaps and detergents have sodium hydroxide.
- Baking soda is a weak base, sodium bicarbonate. It releases CO₂ under high heat, leading to the rise of cakes, bread, etc.
- Ammonia is used for cleaning and in fertilizer.
Conclusion
As you can see, acids and bases aren’t just a random chemistry or biology topic; they have and continue to help improve our living standards across the planet. From the green revolution to better understanding how our body synthesizes protein from amino acids, this topic comes in handy all over.
FAQs:
1. What is an acid, and what is a base?
An acid is a substance that donates protons (in the Brønsted-Lowry definition) or accepts a pair of valence electrons to form a bond (in the Lewis definition). A base is a substance that can accept protons or donate a pair of valence electrons to form a bond. Bases can be thought of as the chemical opposite of acids.
2. What are the differences between acids and bases?
Acids are sour tasting compounds that contain Hydrogen ions (H ) and turn blue litmus papers red. Bases are bitter-tasting compounds that contain hydroxyl ions (OH-) and turn red litmus papers blue.
3. What are five examples of acids and bases?
Acids: Hydrochloric acid, sulphuric acid, nitric acid, lactic acid, hydrobromic acid.Bases: Potassium hydroxide, sodium hydroxide, calcium hydroxide, lithium hydroxide, cesium hydroxide.
4. What are ten examples of bases in everyday life?
- Drain cleaner
- Laundry detergent
- Lubricating grease
- Alkaline batteries
- Soaps and bath products
- Sugar
- Baking soda
- Alcohol
- Hair dye
- Pesticides
5. What are the three types of acids?
The three types of acids are: binary acid, oxyacid, and carboxylic acidBinary acids are hydrogen atoms bonded to nonmetal atoms, written in “H-A” form. Oxyacids have one or more O-H bonds. For example, Sulfuric Acid H₂SO₄. In Carboxylic Acids, only the hydrogen atom in the carboxyl group can be ionized and contribute to acidity.
6. What are the ten examples of acid?
- Hydrochloric acid in gastric juice
- Sulfuric acid
- Nitric acid
- Carbonic acid in soft drinks
- Uric acid in urine
- Ascorbic acid, or more formally known as vitamin C, in fruits
- The citric acid in oranges and lemons
- The acetic acid in vinegar
- The tannic acid in tea and wine
- Tartaric acid in grapes
7. What are five acids and five bases?
Five acids: Lemons, oranges, vinegar, urine, sulfuric acid.
Five bases: Soap, toothpaste, bleach, cleaning agents, limewater.
8. What are the uses of bases?
Milk of magnesia (magnesium hydroxide) is used as an antacid or a laxative by helping correct excess acidity in the stomach.Sodium Hydroxide is used in the manufacture of paper, textiles, and detergents.Calcium Hydroxide is used in whitewashing and making bleaching powder.
9. What are the types of acids and bases?
There are 3 types of acids and bases: Arrhenius, Brønsted, and Lewis.Arrhenius acid dissolves in water to release H ions, and bases release OH- ions.Brønsted acids are compounds capable of donating a proton H . Brønsted bases can accept a proton.Lewis acids can accept an electron pair, and Lewis bases can donate one.
10. What is called acid?
An acid is any substance that tastes sour in water, changes blue litmus paper to red, reacts with some metals to liberate hydrogen, reacts with bases to form salts, and promotes chemical reactions (acid catalysis).
11. What do you mean by base?
The positive or non-acid component of a salt, a substance that, combined with an acid, neutralizes the latter and forms a salt; it is also applied to the hydroxides of the positive elements or radicals and to certain organic bodies resembling them in their property of forming salts with acids.
We hope you enjoyed studying this lesson and learned something cool about Acids and Bases! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun 😎
Sources:
- Bronsted-Lowry Acids and Bases. https://chem.libretexts.org/Courses/Brevard_College/CHE_104%3A_Principles_of_Chemistry_II/07%3A_Acid_and_Base_Equilibria/7.02%3A_Brnsted-Lowry_Acids_and_Bases. Accessed Nov 26, 2021.
- Lewis Concept of Acids and Bases https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Lewis_Concept_of_Acids_and_Bases. Accessed Nov 26, 2021.
- Properties of Acids and Bases. https://courses.lumenlearning.com/cheminter/chapter/properties-of-acids-and-bases/. Accessed Nov 26, 2021.
- Acids and Bases. https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/1.20/primary/lesson/acids-and-bases-in-biology-bio/. Accessed Nov 26, 2021.
- Lewis Acid: Definition, Theory & Examples. https://study.com/academy/lesson/lewis-acid-definition-theory-examples.html .Accessed Nov 26, 2021.
- Three Major Types Of Acids. https://www.tutapoint.com/knowledge-center/view/major-acids. Accessed Nov 26, 2021.