Biochemical Reactions – Energy, and Types of Reactions Study Guide
Introduction:
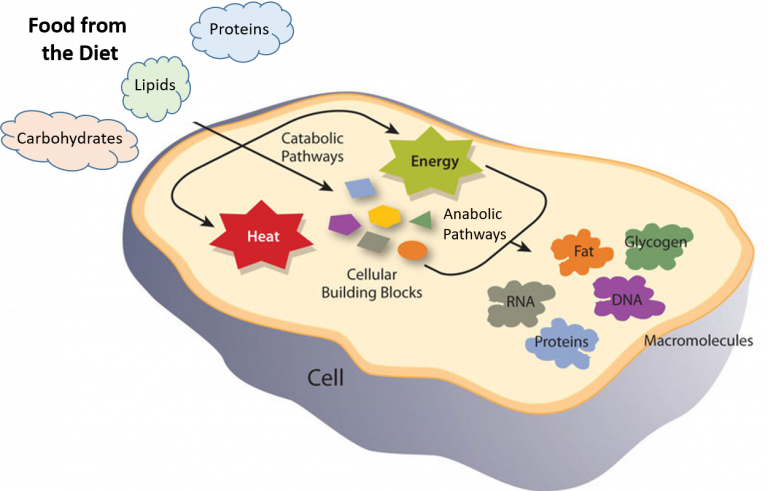
Each chemical reaction in the body needs energy to get started. Energy is a property of matter that is defined as the ability to do work. We get this energy from the food we eat. This is an integral concept steered forward by biochemical reactions.
Chemical reactions that take place inside the cells are termed biochemical reactions. Isomerization reactions, ligation, hydrolysis, and oxidation-reduction reactions are a few examples of biochemical reactions.
It is important to note that all these reactions are enzyme-catalyzed reactions. In other words, various chemical reactions that organisms carry out are collectively called biochemical reactions.
Types of Biochemical Reactions
Anabolic Pathway
It is a metabolic route that necessitates the use of energy to generate bonds. Metabolites are collections of reactants, intermediates, and products. Chemical processes are taking place to construct or produce bigger, more complex macromolecules from smaller micro molecules. The synthesis of sugar is a good example (glucose from CO2 and H2O).
Other examples include fatty acid synthesis from acetyl CoA, protein synthesis from amino acid building blocks, and DNA strand synthesis from nucleotides. These responses happen all the time in the cell and are crucial to the cell’s survival.
Adenosine triphosphate (ATP) and other high-energy molecules like nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate are required for these processes (NADPH). The energy gained will be stored in bigger molecules’ C-C bonds. Examples include photosynthesis, protein biosynthesis, fatty acid synthesis.
Catabolic Pathway
Chemical processes in these routes break down big macromolecules into smaller micro molecules, releasing a considerable amount of bond energy in the process. The decomposition of sugar is a good example (glucose into CO₂ and H₂O).
The energy held in covalent bonds, such as C-C bonds, will be released during these reactions. These pathways can also release energy and produce ATP from energy-storing substances like lipids and glycogen. Examples include glycolysis, Krebs cycle, beta-oxidation of fat.
Amphibolic Pathway
In humans, there are various metabolic pathways. The majority of these metabolic events are related to other reactions and do not occur in isolation. There are two types of pathways: linear and circular.
One pathway’s intermediate (metabolite) might serve as a starting point for another. An example is the breakdown of respiratory substrates such as glucose, protein, and fatty acids. When glucose is used as a respiratory substrate, it first becomes pyruvate, then acetyl CoA.
Similarly, fatty acid beta-oxidation results in the production of acetyl CoA. When a protein is broken down, it produces either pyruvate or acetyl CoA.
When the cell requires glucose, fatty acids, or protein, pyruvate or acetyl CoA can be redirected from the catabolic route for glucose, fatty acids, or amino acid synthesis. As a result, because there is always a connection between the synthesis (anabolic) and breakdown (catabolic) processes, the respiratory system is classified as an amphibolic pathway.
Regulation:
Biochemical pathways interact in a complicated way for proper regulation to occur. Reactions are switched on and off or sped up and slowed down in response to the cell’s immediate needs and overall functions.
Living systems use two complex mechanisms for directing cell metabolism to sustain homeostatic circumstances – Enzymatic control and hormonal control.
Enzymes in Biochemical Reactions
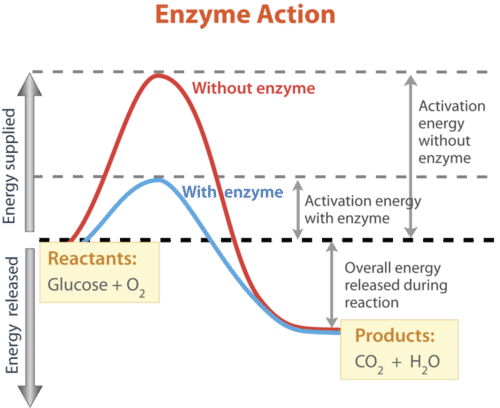
Enzymes are highly effective in accelerating processes. They can catalyze millions of reactions every second. As a result, there might be a significant variation in the rates of biological processes with and without enzymes.
Without an enzyme, a typical biochemical process may take hours or even days to complete under normal cellular circumstances, but it takes less than a second with an enzyme.
A feedback mechanism does the regulation of enzymatic action.
- The feedback mechanism is required since it is the only method to prevent wasting energy in producing end-products that are already plentiful.
- When the final product of a route controls its synthesis rate by inhibiting the initial step, this is known as feedback inhibition.
- The term “enzyme inhibition” refers to the process of preventing an enzyme from operating. An inhibitor binds to the active site of an enzyme and prevents the substrate from attaching to itself, halting the metabolic pathway’s sequence. It’s just a conformational shift.
- The inhibitor merely denatures the enzyme, rendering it inactive, but the binding is only transient. When the inhibitor disengages, the enzyme reverts to its active state and begins to operate on this substrate, reopening the pathways.
The question that commonly comes to mind is – what happens to the enzyme during a metabolic process? Does it get consumed during the reaction, or does it remain intact? When you look at the enzyme’s structure, you will observe several active sites where the substrate binds with the enzyme. The substrate attaches to the enzyme, the reaction occurs, and the final product detaches from the enzymes. Hence, enzymes do not get consumed during the chemical reaction.
Conclusion:
- Chemical reactions that take place inside the cells are termed biochemical reactions. It is important to note that all these reactions are enzyme-catalyzed reactions.
- There are 3 categories of biochemical reactions: catabolic, anabolic, amphibolic.
- Enzymes are highly effective in accelerating processes.
- They can catalyze millions of reactions every second.
- As a result, there might be a significant variation in the rates of biological processes with and without enzymes.
- However, regulation of this process is extremely important. A feedback mechanism does the regulation of enzymatic action.
FAQs:
1. What are biochemical reactions?
Essentially all the chemical reactions taking place in a living organism are known as biochemical reactions.
2. What are the four major types of biochemical reactions?
Four major types of biochemical reactions are oxidation-reduction, hydrolysis, condensation, and neutralization.
3. What are examples of biochemical reactions?
Essentially all the chemical reactions in a living body like Kreb’s cycle, glycolysis, photosynthesis are a few examples of biochemical reactions. Photosynthesis, gluconeogenesis (synthesis of glucose from non-carbohydrate sources, mainly used by the brain), protein biosynthesis, fatty acid synthesis, glycolysis, Krebs cycle, urea cycle, glycogenolysis are some common examples of biochemical reactions.
We hope you enjoyed studying this lesson and learned something cool about Biochemical Reactions – Energy, and Types of Reactions! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App and check out our awesome VR room for this guide – we promise, it makes studying much more fun 😎
Sources
- Types of Biochemical Reactions. https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_Introductory_Biology_(CK-12)/01%3A_Introduction_to_Biology/1.16%3A_Types_of_Biochemical_Reactions. Accessed 25 Nov, 2021.
- Types of Biochemical Reactions. https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/1.16/primary/lesson/types-of-biochemical-reactions-bio/. Accessed 25 Nov, 2021.
- CH103 – Chapter 7: Chemical Reactions in Biological Systems. https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch103-allied-health-chemistry/ch103-chapter-6-introduction-to-organic-chemistry-and-biological-molecules/. Accessed 25 Nov, 2021.