Strong and Weak Acids and Acid Ionization Constant (Ka)
INTRODUCTION
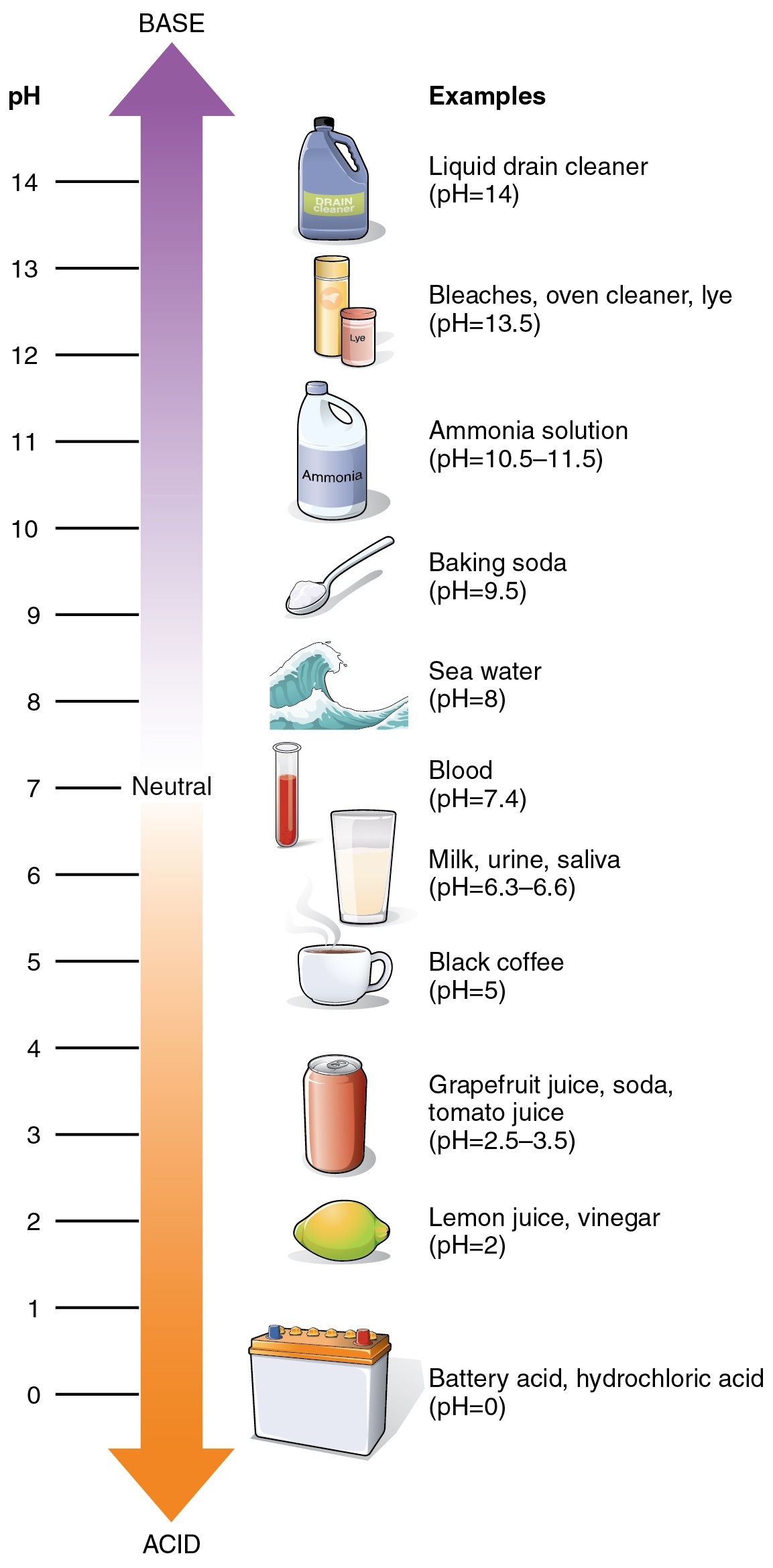
Acids of varying strengths often make up some of our most basic household items. Whether it be a glass of soda or vinegar, coffee or orange, our lives are surrounded by acids. However, acid is of 2 basic types based on their ionization in water or any other solvent. It is categorized into strong or weak acids where the equilibrium constant for the ionization of acid is known as the acid ionization constant.
WHAT ARE STRONG AND WEAK ACIDS?
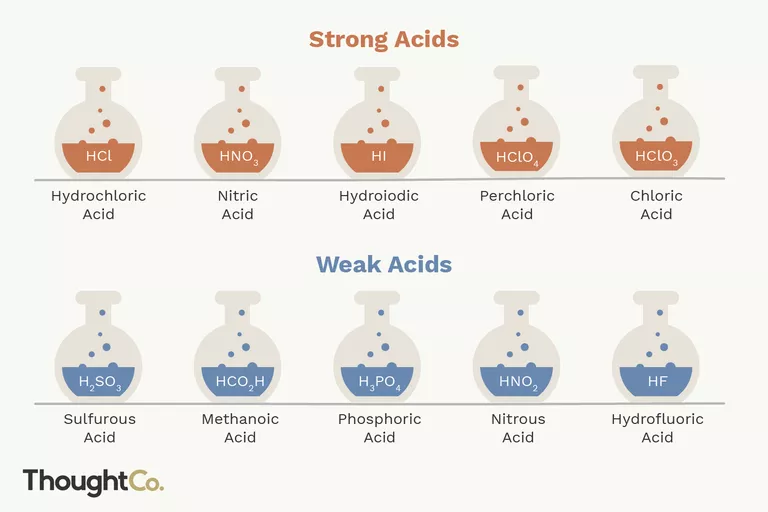
STRONG ACIDS:
Strong acids disintegrate entirely into their constituents’ ions in water, releasing one or more protons (hydrogen cations) per acid molecule.
HCl → H+ + Cl-The creation of positively charged hydrogen ions and the reaction arrow that exclusively points to the right should be noted. The reagent (acid) is completely ionized (dissociated into its constituents ions) and transformed into a product.
WEAK ACIDS:
In water, weak acids may not fully disintegrate into their constituent ions.For instance, HF disintegrates into H+ and F- ions however, some HF persists in solution, making it a weak acid. Weak acids outnumber strong acids by a large margin. Organic acids, for the most part, are weak acids.
DISTINGUISHING BETWEEN STRONG AND WEAK ACIDS
To evaluate whether an acid is strong or weak, evaluate the acid equilibrium constant Ka or pKa. Strong acids have a high Ka value or perhaps a small pKa value, whereas weak acids have a very low Ka value or perhaps a big pKa value. Now let’s discuss what acid equilibrium constant is.
ACID DISSOCIATION CONSTANT (Kₐ)
-
An ionization constant (abbreviated as K) is a value that depends on the equilibrium between ions and non-ionized molecules in a solution or medium.
-
The acid dissociation constant (Kₐ) measures an acid’s strength in solution. The corresponding dissociation process of acid in an aqueous solution has an equilibrium constant of Kₐ:
HA (aq) ⇌ H+(aq) + A−(aq) Kₐ= [H+] [A−]/[HA] -
Weak acids, or acids which do not entirely dissolve in solution, are most commonly connected with acid dissociation constants. This is because strong acids are thought to ionize entirely in solution hence their Ka values are quite high.
CONCLUSION
- Based on their ionization in water, acids are classified as either strong or weak.
- In an aqueous solution, a strong acid is completely ionized (completely dissociates into constituent ions).
- Only a minute amount of ionization (dissociation into constituent ions) occurs in weak acids.
FAQs:
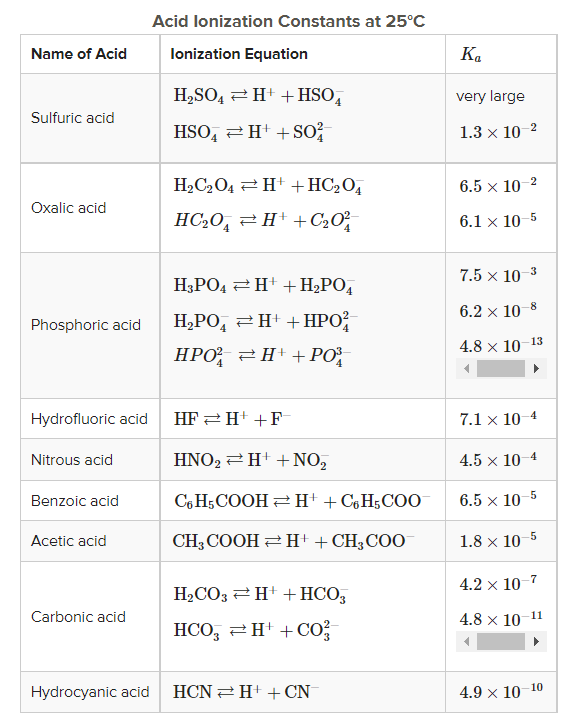
1. Which is the weakest acid depicted in the table above?
Comparing the different ionization constants (Kₐ) of different acids, Hydrocyanic acid seems to be the weakest acid amongst all because it has the smallest Kₐ.
2. What is the ionization constant?
An ionization constant (abbreviated as K) is a value that depends on the equilibrium between ions and non-ionized molecules in a solution or medium. The ratio of products to reactant molecules increased to the corresponding stoichiometric powers or the ratio of the concentration of product to the reactant.
3. What is the difference between a strong and weak acid?
A strong acid is one that entirely ionizes in an aqueous solution. Once mixed with water, it always releases a proton (H+). A weak acid is one that only partially ionizes in a solution. When mixed with water, it barely lets out a portion of its (H+) atoms.
4. Which acid is of weak strength?
Weak acids are partially ionized (incomplete dissociation into constituent ions) in water or any other solvent.
We hope you enjoyed studying this lesson and learned something cool about Strong and Weak Acids and Acid Ionization Constant (Kₐ)! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Strong and Weak Acids and Acid Ionization Constant (Kₐ). https://www.ck12.org/c/chemistry/strong-and-weak-acids-and-acid-ionization-constant-ka/lesson/Strong-and-Weak-Acids-and-Acid-Ionization-Constant-Ka-CHEM/. Accessed 7 Feb 2022.
- Acid Dissociation Constant (Kₐ). https://courses.lumenlearning.com/introchem/chapter/acid-dissociation-constant-ka/. Accessed 7 Feb 2022.
- List of Strong and Weak Acids. https://www.thoughtco.com/list-of-strong-and-weak-acids-603642 accessed 7 Feb 2022