Strong and Weak Bases and Base Ionization Constant (Kb) Study Guide
INTRODUCTION:
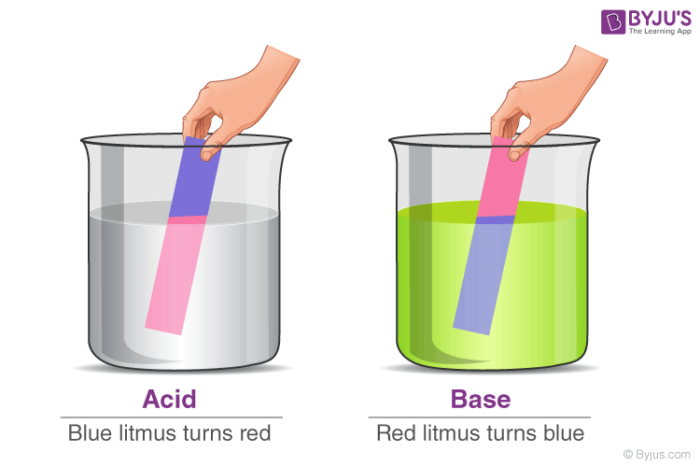
You must have learned that when a litmus paper is placed in an acid or base, it changes color in your classes. This is also the indicating test to determine whether the given liquid is acid or base, or just water! Has it ever occurred to you what happens after identifying the given solution? Are all the acids or bases the same? Do all bases have the same properties or strength? The answer is a ‘no, all bases are not the same.!
They all have different strengths and different properties. There are 2 basic types of bases on the basis of their strength- weak base and strong base. Note here that we have used the term “strength of the base.” What is this really? Let’s find out!
STRENGTH OF BASE:
The capacity of a species to receive H+ from another species is its base strength (Brønsted-Lowry theory). The higher a species’ capacity to receive an H+ from another species, the stronger its basic strength becomes.
Kb OF STRONG BASES
- A base is a chemical that may receive hydrogen ions (H+) or give a couple of valence electrons more broadly. A weak base is a chemical that does not completely ionize in an aqueous solution.
- In chemistry, there are scales for determining how acidic or basic a solution is and the concentration of acids and bases.
- The basic dissociation constant (Kb) is one such scale. In water, the base dissociation constant is a measurement of how thoroughly a base dissociates into its constituent ions.
- A significant Kb value implies a strong base’s high amount of dissociation. A lower pKb value indicates a stronger base.
- In an aqueous solution, a weak base ionizes just minimally. Keep in mind that a base is a material that takes a hydrogen ion from another chemical. When a weak base like ammonia dissolves in water, it absorbs an H+ ion from the environment, generating the hydroxide ion and the base’s conjugate acid, the ammonium ion.
NH₃ (aq)+ H₂O (l) ⇄ NH+₄ (aq) + OH− (aq)
An equilibrium equation may be developed for the interactions of weak bases and water. Water is not included in the equation since its concentration is exceedingly high and nearly constant. The equilibrium constant for the ionization of a base is known as the base ionization constant (Kb). The Kb equation for ammonia is:
Kb = [NH+₄] [OH−] / [NH₃]
FEW COMMON USES OF STRONG BASES:
Strong bases have a slick, soapy texture and a high pH value. Cleaning solutions and antacids both include strong bases. Sodium hydroxide is a common element in drain unclogging solutions. Check the sodium hydroxide in the list of active components the next time you buy a bottle of chemical drain cleaner.
FEW COMMON USES OF WEAK BASES:
Because of their involvement in buffer solutions, weak bases have major uses in biological investigations, chemistry processes, and physiological objectives. Weak bases can also be employed to catalyze processes like the production of enolates, as seen in the diagram below:
CONCLUSION:
- A weak base cannot fully ionize or take hydrogen ions in an aqueous solution because it can receive hydrogen ions (H+) or, more broadly, contribute a pair of valence electrons.
- The base dissociation constant, Kb, is related to the acid dissociation constant in that it mathematically quantifies the relative strength of the base. Weaker bases have lower Kb values.
FAQs:
1. How do you know if a Kb base is strong or weak?
A significant Kb value implies a strong base’s high amount of dissociation. A lower pKb number denotes a more powerful base.
2. Why do strong bases have large Kb values?
The basic dissociation constant is Kb. In water, the base dissociation constant is a measurement of how thoroughly a base dissociates into its component ions. A significant Kb value implies a strong base since the base has fully dissociated.
3. What is the kb of weak bases?
The base dissociation coefficients are calculated in the same way as the acid dissociation values are calculated. A strong base has a small pKb value, while a weak base has a great pKb value.
We hope you enjoyed studying this lesson and learned something cool about kb of strong bases! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Significance of Kb. https://www.ck12.org/c/chemistry/strong-and-weak-bases-and-base-ionization-constant-kb/lesson/Strong-and-Weak-Bases-and-Base-Ionization-Constant-Kb-CHEM/. Accessed 26 Jan 2022.
- Strength of Bases. https://courses.lumenlearning.com/boundless-chemistry/chapter/strength-of-bases/. Accessed 26 Jan 2022.