Atom Size Study Guide
INTRODUCTION
Have you ever wondered what your body is made up of? What about the ball you play soccer with? Ever wondered what plants are made of? You might have heard the word ‘atom,’ but do you know what it is? Atoms are considered the basic units of matter and everything on the earth is made up of atoms except for energy.
Where does the word atom come from? Atom comes from the classic Greek word for indivisible because it was thought then that atoms are the smallest universal thing and they cannot be further divided.
However, you must have heard of electrons, protons, neutrons? But what are they? And, how are they related to atoms? Atoms can be divided, and they can be divided into subatomic particles called protons, electrons, and neutrons which can again be divided into quarks.
DEFINING NUCLEUS
Atoms were formed after the Big Bang theory, which happened 13.7 billion years ago. As the earth cooled down, it led to the formation of quarks and electrons. Quarks then came together to form protons and neutrons. Protons and neutrons are a lot heavier than electrons and reside within the atom’s nucleus. Protons and neutrons have almost the same mass.
Ernest Rutherford first discovered the nucleus in 1911, as stated by the American Institute Of Physics. In 1920, he was the first one to propose the name proton for positive charged atomic particles. He also stated that there was a neutral particle which later on, his student, James Chadwick, confirmed in the year 1932.
WHAT ARE PROTONS?
- Protons are present within the atomic nucleus and are positively charged.
- Rutherford had conducted an experiment with cathode-ray tubes and had ended up discovering protons.
- For each element, the number of protons present is different. For example, carbon has six protons while oxygen has eight. The designated number of protons determines the chemical behavior of the element.
WHAT ARE ELECTRONS?
- Electrons are much smaller when compared to neutrons and protons, almost 1,800 times smaller.
- Electron was discovered by Joseph John Thomson.
- Electrons were formally termed ‘corpuscles’ and had a negative charge and are attracted to the positively charged protons.
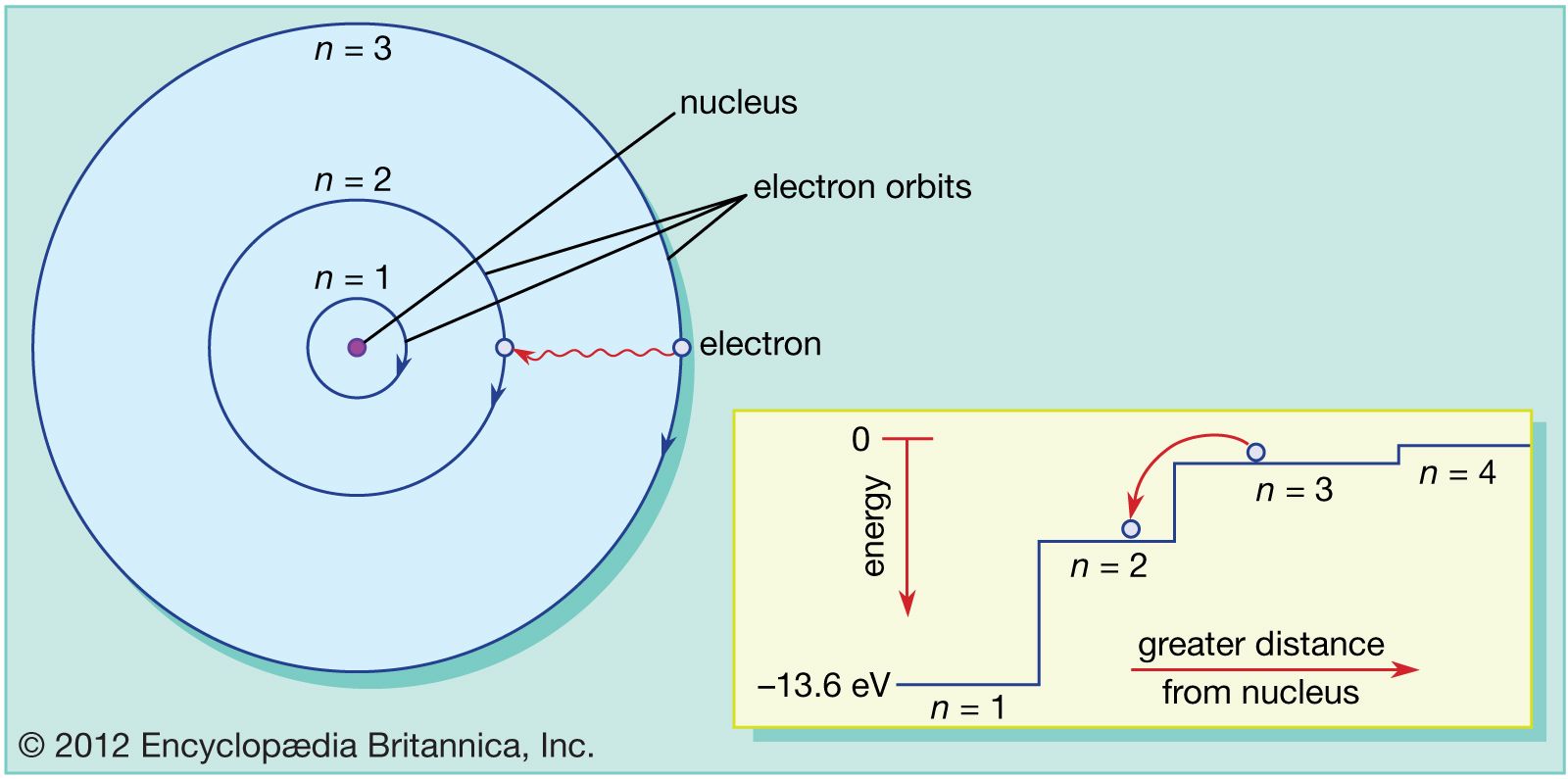
WHAT IS ATOMIC SIZE?
Atomic size is the distance between the atom’s outermost shell and that to the center of the nucleus.
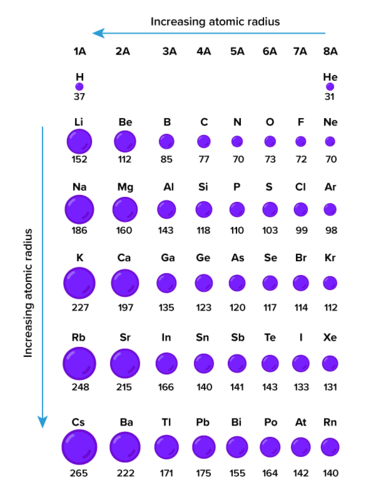
WHAT IS ATOMIC RADIUS?
Atomic radius is half of the distance between the adjacent atoms of the same element in a molecule.
- When two atoms combine, we can possibly determine the size of atoms by checking the distance between the atoms.
- Another method by which one could check the atomic size would be by forming a single covalent bond between two atoms and then checking the distance.
How is atomic radius determined? It is a complicated process and is usually done by X-ray or spectroscopy methods.
CONCLUSION
- The nucleus is made of protons and neutrons.
- Proton is positively charged, and the neutron is neutral.
- Electrons are negatively charged and attracted towards protons.
- Atomic size is the distance between the atom’s outermost shell and that to the center of the nucleus.
- When two atoms combine, we can possibly determine the size of atoms by checking the distance between them.
FAQs
1. Do atoms have a size?
Yes, atoms do have sizes.
2. What is smaller than an atom?
Atoms can be divided into protons, neutrons, and electrons.
We hope you enjoyed studying this lesson and learned something cool about Atom Size! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Atomic Mass and Isotopes: https://www.britannica.com/science/atom/Atomic-mass-and-isotopes. Accessed 28 Feb 2022.
- Atomic Radius in Periodic Table: https://byjus.com/chemistry/atomic-radius-in-periodic-table-in-basic-chemistry/. Accessed 28 Feb 2022.
- What is an Atom: https://www.livescience.com/37206-atom-definition.html. Accessed 28 Feb 2022.