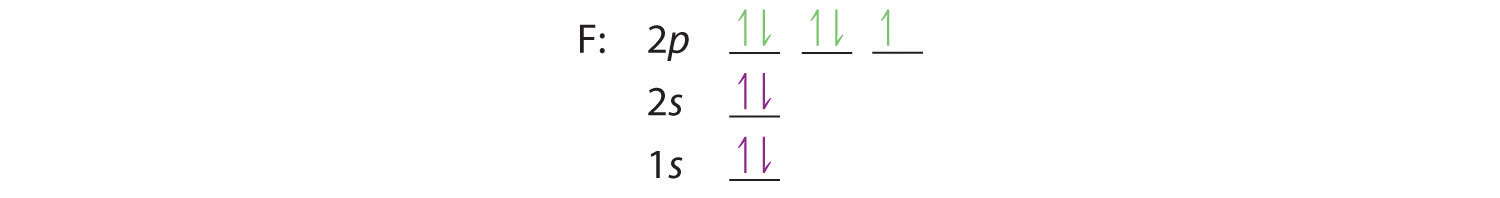Electron Configuration Study Guide
Introduction
An atom consists of subatomic particles like electrons, protons, and a nucleus. The electrons are negatively charged, and they revolve around the nucleus in a designated orbit. As we move higher on the periodic tables, the elements have electrons arranged in orbits far away from the nucleus.
The shape of the orbits is also different. The electron configuration refers to the manner in which electrons are arranged in orbital shells and subshells. Usually, when the electrons are in the ground state.
However, we can also represent ionization status using electron configuration rules. The chemical property of an element can be determined by electron configuration in the valence shells, which makes this lesson quite important.
HOW TO ASSIGN ELECTRON CONFIGURATION?
To write an electron configuration, we follow two very important principles.
- First, we apply the Aufbau principle or the building up principle, which indicates that we must identify the total number of electrons in an element.
- Next, we start assigning these electrons from the lowest-energy orbital shells. The second principle that we must follow closely aligns with the Pauli Exclusion principle
- The exclusion principle states that every orbit can hold a maximum of two electrons with spin up and spin down. Therefore, if one electron is assigned as a spin-up (ms = +1/2) electron, the other electron must be a spin-down (ms = -1/2) electron. When an orbital shell is full, it is represented by the symbol ↑↓. (Where ms is spin quantum number)
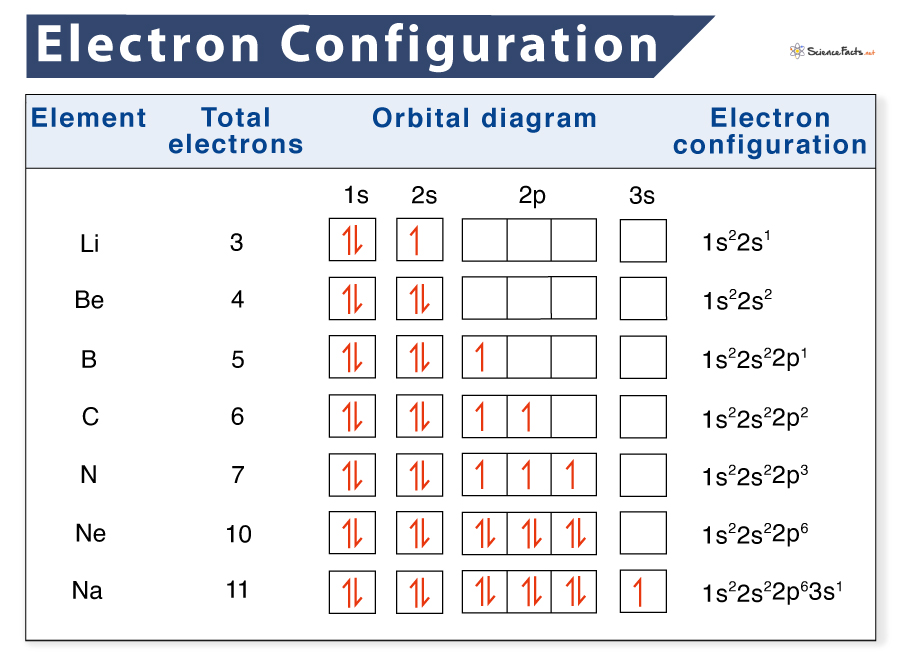
Let’s take the example of a Hydrogen atom to understand this.
- Hydrogen has one electron with almost the same energy level as the nucleus. Therefore, the energy level is represented by the roman numeral 1.
- This electron revolves around the nucleus in an s-shaped orbit. Therefore, the orbital-shell notation comes out as 1s.
- Now, we write the number of electrons in the subshell in the superscript.
- So, the final spdf notation for Hydrogen comes out as 1s1.
Similarly, let’s try to figure out the configuration of Helium using the principles mentioned above:
- Helium has an atomic number of two, which means it carries two electrons.
- We follow the same steps as Hydrogen and start by filling the lowest energy orbital shell.
- However, the 1s orbital can hold two electrons with opposite spins.
- So, the final configuration comes out to be 1s2.
As we move higher on the periodic table, the atomic number increases, so it becomes tedious to write the repetitive inner shell configuration. For this reason, we use noble gas configuration to write the electron configuration of cadmium, lead, etc.
THE ELECTRON CONFIGURATION OF DIFFERENT ELEMENTS
Let’s take a look at the electron configuration of some popular elements:
1. Fluorine: The noble gas configuration for fluorine is 1s22s22p5.
2. Lead: The atomic number of Pb is 82, and Pb noble gas configuration is (Xe)6s24f145d106p2.
3. Cadmium: We use Krypton’s inner shell configuration to write the electron configuration for cadmium. The atomic number of cadmium is 48, and the cadmium electron configuration is (Kr)4d105s2.
Conclusion
- The arrangement of electrons of an element in orbit shells and subshells is known as electron configuration.
- The number of electrons present in the valence shell also determines the chemical property of an element.
- The electron configuration mainly captures the electron arrangement in its ground state.
- There are two principles that must be strictly followed when writing an electron configuration, the Aufbau principle and Pauli’s exclusion principle.
FAQs
1. What is the noble gas notation for fluorine?
(He)2s22p5
2. What is the noble gas notation for Fe?
(Ar)3d64s2
We hope you enjoyed studying this lesson and learned something cool about Electron Configuration! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
Sources
- Electron Configuration of Atoms: https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1402%3A_General_Chemistry_1_(Kattoum)/Text/7%3A_The_Structure_of_Atoms_and_Periodic_Trends/7.3%3A_Electron_Configurations_of_Atoms.Accessed 9th March 2022.
- Electron Configuration of Iodine and Lead:https://www.ck12.org/c/chemistry/noble-gas-configuration/lecture/Electron-Configuration:-Iodine-I-and-Lead-Pb/.Accessed 9th March 2022.