Activity Series Study Guide
Have you ever wondered why different metals and nonmetal elements react with each other? Why do some of the elements in the periodic table not react at all? All of these are very thoughtful questions. We know due to some of the chemical reactions, all the elements react to form new compounds. But, how to understand which elements will react with the other or which one will replace and take its place?
Well, it can be difficult to understand how an element will react and in which conditions; hence, to know the reactivity of metals, the activity series is developed.
WHAT IS AN ACTIVITY SERIES?
The Activity Series is developed to determine the reaction of metals and non-metals elements with different elements in different conditions. It helps to determine the nature of the reaction and which element will displace the other element in a chemical reaction. The activity series is a set of different elements like metals and non-metals listed in decreasing order of their reactivity. Here is the activity series chart
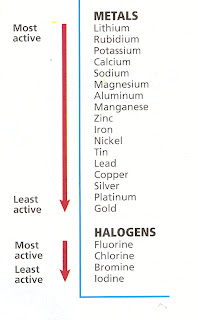
WHAT IS THE ACTIVITY SERIES OF METALS?
The reactivity series of metals is a chart listing metals in order of decreasing reactivity of the elements. In general, the more reactive a metal is, the more vigorously it reacts with other metals elements. The faster the reaction, the faster it loses electrons to form positive charges or positive ions.
The activity series of metals was developed so that it becomes easier for us to understand which metals are more reactive and which ones will react in any medium like sodium, magnesium, etc. Also, to visualize which metals take time to react to replace the hydrogen atoms like cobalt, nickel, tin, etc. Some of the elements do not react at all; these are the metals that are under hydrogen, like silver, gold, and platinum.
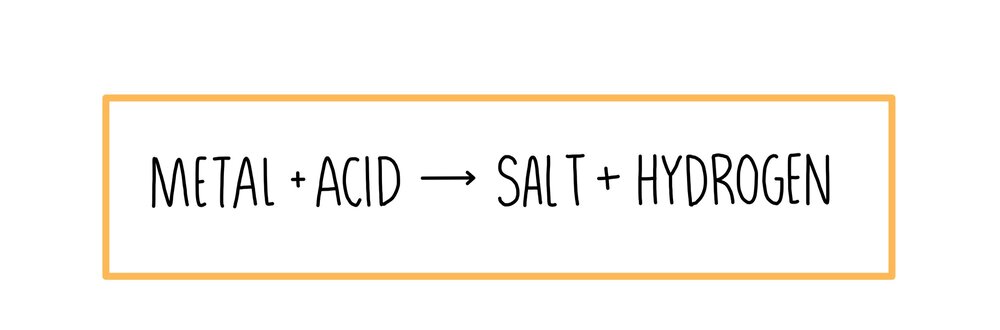
STEPS TO PREDICT PRODUCTS OF A REACTION
We need to understand the Activity Series to determine and predict the products of the reaction. Here are the following steps:
Step 1: Check the elements in the reaction. See where the elements are situated in the Activity Series. Also, check the reaction of the elements if it is highly active or not.
Step 2: Solve or determine the reaction. As we know that the highly reactive elements will replace the less reactive elements; hence, you will get the products of the chemical reaction.
Example:
Zn (s)+H2SO4 (aq) → ZnSO4+H2↑
Ag (s)+HCl(aq) → No Reaction
FAQs
1. What is an activity series used for?
The activity series is used to determine how an element will react when it is kept in water, acid, or solutions containing different ions of different metals.
2. What is an activity series example?
An Activity series consists of lists of elements according to their decreasing order of reactivity. One of the biggest examples of activity series is the noble metals like silver, gold, and platinum, which are found at the bottom of the metal activity series.
It shows that it does not react with almost anything, so it is safe to use as ornaments or jewelry. Another example is the reaction of lithium with cold water as it displaces hydrogen from the water. Lithium is a highly active element that reacts with steam and acids.
3. What determines the activity series?
The activity series determines the capability to displace hydrogen gas from water and other solutions like acid or ionic solutions.
We hope you enjoyed studying this lesson and learned something cool about the Importance of Activity Series! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>