Single Replacement Reactions Study Guide
INTRODUCTION
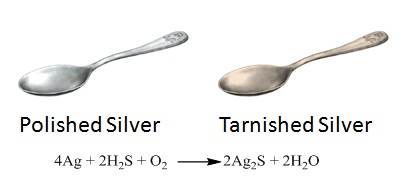
Tarnish is a chemical reaction that occurs whenever silver metal combines with hydrogen sulfide gas produced by various industrial operations or as a consequence of rotting animal or plant elements, as demonstrated in the spoon here.
A variety of polishes may be used to remove the tarnish, but the technique also loses a little bit of silver in the process. What is of interest, is the type of reaction that is taking place. Silver removes hydrogen from the sulfide and forms silver sulfide. This type of reaction is also known as a single replacement reaction.
REPLACEMENT REACTION
- When elements in a compound swap locations, this is called a replacement reaction. Ions (electrically charged counterparts of atoms) or ionic compounds are involved in this sort of reaction.
- In most cases, a more reactive component substitutes a less reactive element, and the less reactive component is liberated from the combination.
SINGLE REPLACEMENT REACTION
-
A single replacement reaction happens whenever one element replaces the other in a single component. The general expression for this sort of reaction is:
A + BC → B + AC -
When potassium combines with water, it is an example of a single substitution reaction. Potassium hydroxide, a white solid chemical, is formed, and hydrogen gas is released.
2K + 2H₂O → 2KOH + H₂ -
According to the expression, a potassium ion substitutes one of the hydrogen atoms within every water molecule in this process. Because potassium is a powerful oxidizing group 1 alkali metal, it reacts violently with water.
HYDROGEN REPLACEMENT REACTION
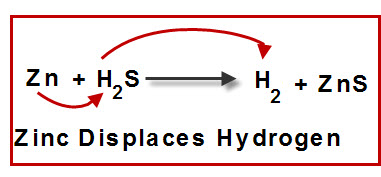
Many metals readily react with acids, and one of the results of the process is hydrogen gas. Aqueous zinc chloride and hydrogen are formed when zinc combines with hydrochloric acid.
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) The hydrogen in the acid is substituted by an active metal in a hydrogen replacement process.
PREDICTING IF A SINGLE REPLACEMENT REACTION WILL OCCUR
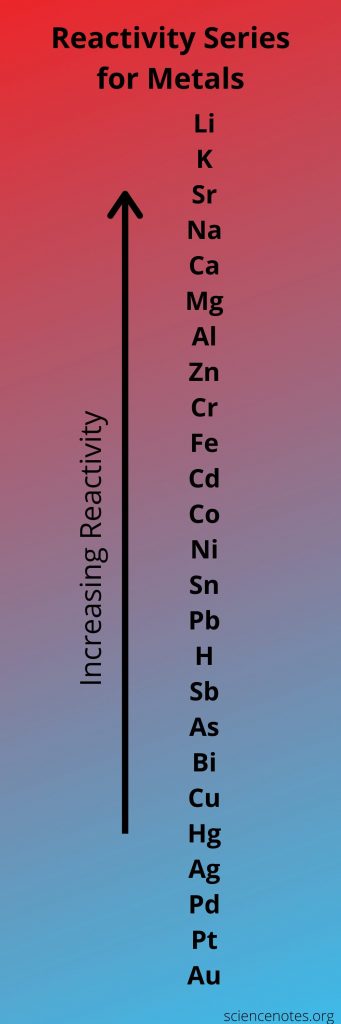
For specific types of reactions, such as single replacement reactions, the reactivity series—also known as the activity series—ranks constituents in a sequence of their reactivity. In the reactivity series, the more reactive components will displace the less reactive elements, but the other way around is not possible.
CONCLUSION:
- A single replacement reaction happens whenever one element replaces the other in a single component.
- Many metals readily react with acids, and one of the results of the process is hydrogen gas.
- Aqueous zinc chloride and hydrogen are formed when zinc combines with hydrochloric acid.
FAQs:
1. Is Zn + 2HCl = ZnCl₂ + H₂ a single replacement reaction?
Yes, zinc reacting with hydrochloric acid giving zinc chloride and hydrogen gas as products, is a typical example of a single replacement reaction.
2. What happens when Zn reacts with HCl?
When zinc reacts with HCl, zinc displaces hydrogen from hydrochloric acid and form zinc chloride. Hydrogen gas is released as a by-product.
We hope you enjoyed studying this lesson and learned something cool about Zn and HCl Single Replacement! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Single Replacement Reactions: https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/11.7/primary/lesson/single-replacement-reactions-chem/ accessed 5 Feb 2022
- Single Replacement Reactions: https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/types-of-chemical-reactions/a/single-replacement-reactions accessed 5 Feb 2022
- Single Replacement Reactions: https://sciencenotes.org/single-replacement-reaction-definition-and-examples/ accessed 5 Feb 2022