Hybrid orbitals – sp and sp² Study Guide
INTRODUCTION
We all love a delicious piece of cake 🍰 every now and then, but do you know how we bake a cake? Starting with all the ingredients, we mix them in the required proportions, and then we bake the batter in the oven for a given period. And the result? A mouth-wateringly delicious cake 😋!
In some ways or the other, hybridization is like baking a cake. We start with all the atomic orbitals we require, and the result is a hybrid orbital that will take place in bonding. Let’s look at a few examples of these hybridized orbitals.
sp HYBRIDIZATION
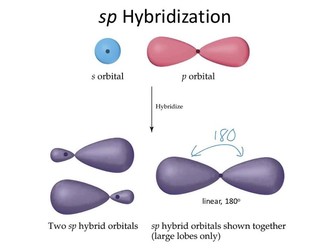
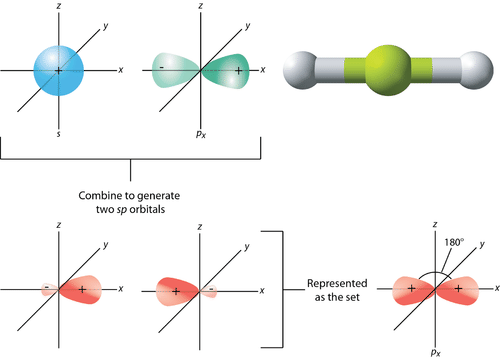
- Whenever one s and one p orbital in the same primary shell of the atom combine to generate 2 new equivalent orbitals, this is known as sp hybridization. sp hybridized orbitals are the new orbitals that develop. It produces 180-degree linear molecules.
- 1 ‘s’ orbital and also 1 ‘p’ orbital of comparable energy are mixed to form a new hybrid orbital termed as a sp hybridized orbital.
- Diagonal hybridization is another name for sp hybridization.
- Each sp hybridized orbital has the same proportion of s and p characters – 50 percent s and 50 percent p.
sp² HYBRIDIZATION
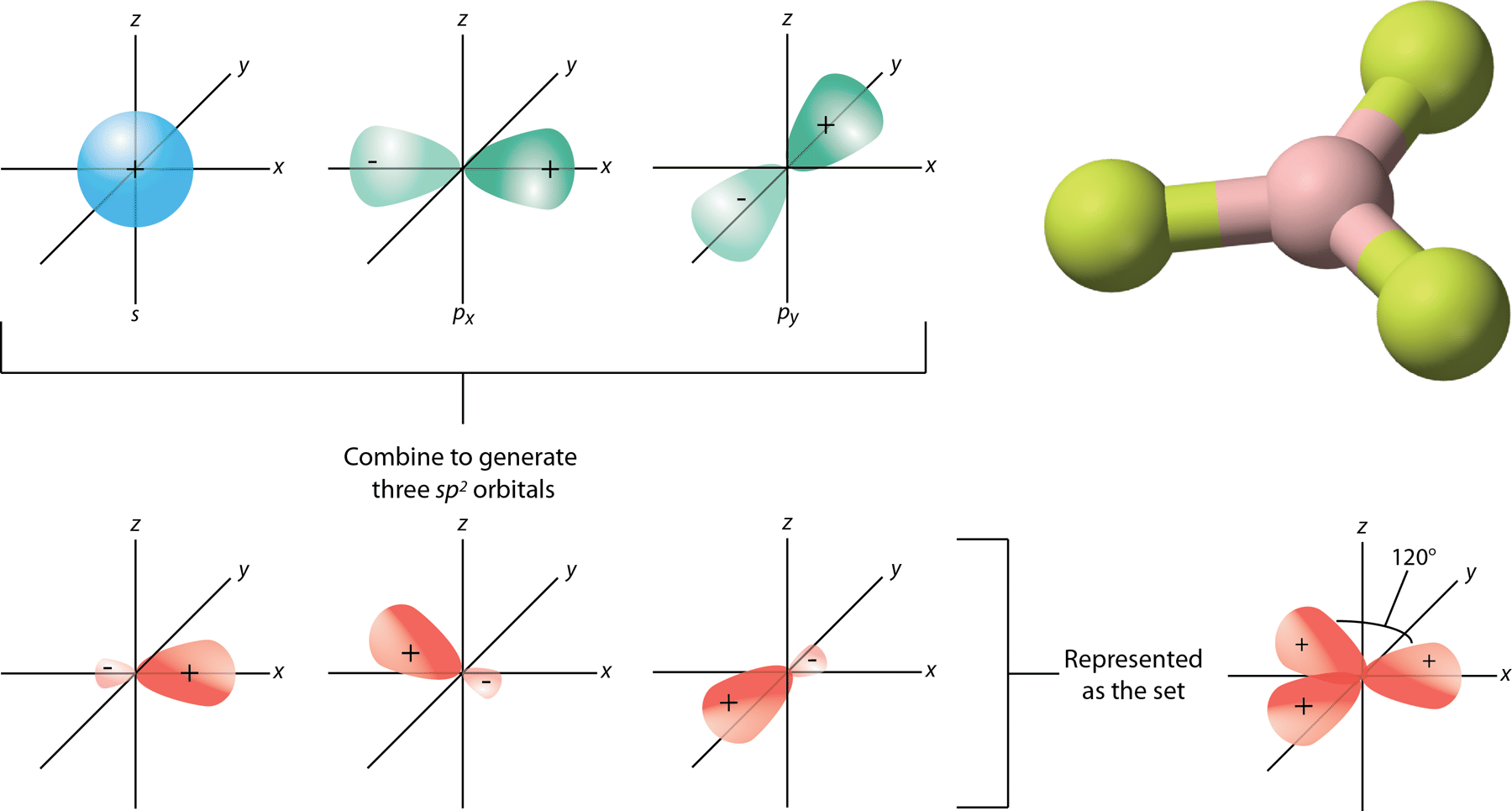
- Whenever 1 s and 2 p orbitals of the very same shell of an atom combine to generate three equivalent orbitals, this is known as sp² hybridization. Sp² hybrid orbitals are indeed the new orbitals that have been created.
- Trigonal hybridization is another name for sp² hybridization.
- It entails combining 1 s orbital with two ‘p’ orbitals of equivalent energy to form the sp² hybrid orbital.
- A trigonal geometry blend of s and p orbitals is retained at 120 degrees.
- All three hybrid orbitals stay in the same plane and form a 120° angle with one another. The hybrid orbitals have a 33.33 percent ‘s’ character and a 66.66 percent ‘p’ component in each of them.
A planar triangle form is seen in structures where the center atom is connected to three other atoms and is sp² hybridized.
CONCLUSION:
- Hybridization of paired electrons allows them to engage in covalent bonding.
- Diagonal hybridization is another name for sp hybridization.
- Trigonal hybridization is another name for sp² hybridization.
FAQs:
1. How many orbitals are in sp?
The sp set consists of two similar orbitals that are 180 degrees apart. The two electrons previously in the s orbital have been dispersed to the two half-filled sp orbitals.
2. What is sp sp² and sp³?
One s and one p atomic orbital are mixed in sp hybridization, one s and 2 p atomic orbitals are mixed in sp² hybridization, and one s and 3 p atomic orbitals are mixed in sp³ hybridization.
3. What is the shape of sp orbitals?
The word sp-hybridization refers to the mixing of one s- and one p-orbital. The Be atom, for instance, undergoes sp-hybridization in BeF₂. It has a linear shape, and the bond angle is 180 degrees.
We hope you enjoyed studying this lesson and learned something cool about hybrid orbitals – sp and sp²! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Hybrid Orbitals – sp and sp2 https://www.ck12.org/c/chemistry/hybrid-orbitals-sp-and-sp2/lesson/Hybrid-Orbitals-sp-and-sp2-CHEM/. Accessed 18 Feb 2022
- Hybridization. https://byjus.com/jee/hybridization/. Accessed 18 Feb 2022
- Sp Hybridization. https://courses.lumenlearning.com/introchem/chapter/sp-hybridization/. Accessed 18 Feb 2022