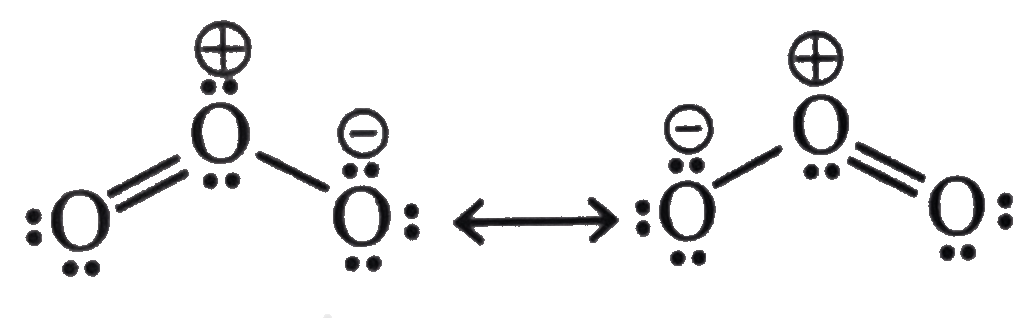Resonance Study Guide
INTRODUCTION
It is critical for every one of us to live and spend time in a caring, secure, and stable environment. We all wish to feel like we fit, valued, and cherished, and we may get that feeling by expressing our emotions with others. The more people we share our feelings with, the more stable we get in our community. Likewise, molecules demonstrate their stability by a resonance structure that grows in proportion to the number of structures they contain.
WHAT IS RESONANCE IN CHEMISTRY?
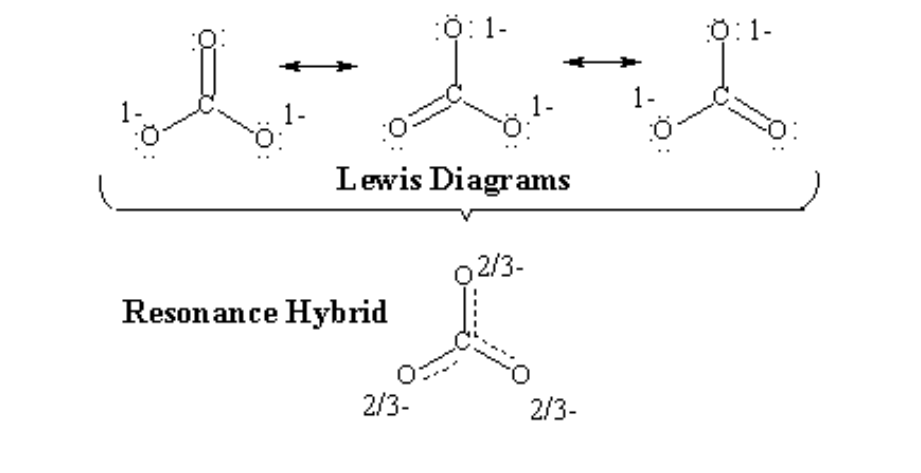
The electrons and bonds of a molecule can be seen using Lewis dot structures. Yet, some compounds have many participating or “resonance” configurations since a single Lewis structure cannot capture all bonding configurations. In chemistry, resonance refers to the concept that electrons are delocalized or circulate easily within the molecule, allowing for the possibility of different configurations for a single molecule. Resonance structures are created when a chemical or molecule represents two or more separate hybrid configurations with different electron positions.
Creating a Lewis structure for every relevant resonance structure can be visualized; however, it is worth remembering that none of these structures can be seen in reality. That is, the molecule does not actually switch between different configurations; instead, the genuine structure is a close approximation of each one of them.
Note: When sketching resonance structures, keep in mind that only the electrons should be moved; the atoms must remain in the same place. The placing of opposite charges on certain atoms is sometimes used in resonance structures.
An example for resonance structure can be:
Resonating structures of ozone (O₃)
The core oxygen atom of the ozone (O₃) molecule is individually connected with one oxygen atom and doubly bound to the other. Although this molecule has no net charge, the Lewis structures reveal a positive unit charge on the core oxygen and a negative unit charge on the singularly linked oxygen. The two ozone ( O3) resonance structures are seen below.
CONCLUSION:
- Resonance is a feature of a molecule that can be viewed as numerous structures with only minor differences in electron distribution.
- The net charge of resonance structures will be the same.
- The molecule of ozone has two resonating structures.
FAQs
1. Does ozone have resonance?
Yes, the element of ozone does process the property of resonance.
2. How many resonance structures can ozone form?
Ozone can form a total of two resonating structures.
3. What is the concept of resonance?
In VBT (Valence Bond Theory), resonance, also known as mesomerism, is a process of describing bonding in specific molecules by combining multiple attributing structures, also recognized as resonance structures, into a resonance hybrid.
We hope you enjoyed studying this lesson and learned something cool about the Resonance! Join our Discord community to get any questions you may have answered and to engage with other students just like you! We promise it makes studying much more fun! 😎
SOURCES:
- Resonance: https://www.vedantu.com/question-answer/resonance-structures-can-be-drawn-for-ozo-class-11-chemistry-cbse-5fd8cb4b609c0e2b76a2ac4b. Accessed 24 Feb 2022.
- Resonance: https://en.wikipedia.org/wiki/Resonance_(chemistry). Accessed 24Feb 2022.