Single Covalent Bonds Study Guide
INTRODUCTION
Have you ever wondered what keeps two elements bonded together? The periodic table will always fascinate you with all the different elements which form different bonds with each element. One of the common bonds is the covalent bond.
A covalent bond can be defined as the atomic linkage which forms due to the result of sharing two electrons from each atom. The covalent bond has three types: single covalent bond, double covalent bond, and triple covalent bond.
Let us learn more about single covalent bonds!
WHAT IS A SINGLE COVALENT BOND? WHY DOES IT FORM?
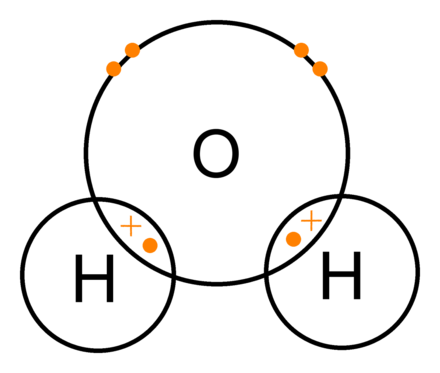
A single covalent bond can be defined as the result of two atoms sharing two electrons to form a chemical bond. Hence, it can be said that a covalent bond in which only one pair of electrons is shared is known as a single covalent bond. With sharing a bond, a molecule or compound is formed in a new chemical reaction. It increases the intermolecular force as it shares one electron from each atom. There is only one line between the two atoms.
A covalent bond forms when the discrepancy between the electronegativities of two atoms is too small for an electron transfer to occur to form ions. It is mainly formed between two nonmetals. Neither of the atoms involved in a covalent bond has enough energy to tear an electron out from the other atom, like the same in ionic bonds. But the atoms still want to form a full octet; hence, they share their valence electrons, and covalent bonds are formed.
HOW IS A SINGLE COVALENT BOND FORMED?
In a single covalent bond, each atom donates with one electron. So if two non-metals need to form a bond, they need to send one electron from each side and form a bond. Many atoms donate to form a stable compound, and the bond also helps to achieve the duplet, octet, etc. For example, in methane, there are clearly four single covalent bonds. Each of which stands for a pair of electrons. Single covalent bonds can be represented by single lines.
FAQs
1. What do single covalent bonds share?
Single covalent bonds share one pair of electrons to form the bond.
2. Why are single covalent bonds the longest?
Single bonds are the longest as interatomic attraction is greater in the two other types. The expansion in component bonds is the reason for this attraction increase as more electrons are shared between the bonded atoms.
3. What elements can only have a single bond?
The hydrogen atom and the halogen atoms form only one covalent bond with the other atoms as a steady neutral compound. However, the carbon, nitrogen, and oxygen atoms can bond to more than one atom.
4. What is the amount of force required to break a single covalent bond?
The specific energy of a covalent bond is 1 electron-Volt, and the typical distance over which the bond stays is normally 0.1 nanometer. Thus, the force required to break a covalent bond is on the order of 1 eV/0.1 nm ~ 1600 pN.
We hope you enjoyed studying this lesson and learned something cool about Covalent Bonds! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Single Covalent Bonds: https://www.ck12.org/c/chemistry/single-covalent-bonds/lesson/Single-Covalent-Bonds-CHEM/?referrer=concept_details.Accessed 7th March 2022.
- Covalent Bond: https://www.sciencedirect.com/topics/chemistry/covalent-bond. Accessed 7th March 2022.