Calculating Standard Cell Potentials Study Guide
INTRODUCTION:
Can you imagine your life without batteries? Look around you. Nearly every other appliance that we see runs on batteries. Maybe it’s your alarm clock or the remote for your television. The discovery of batteries has made our lives a lot easier. The smartphones we carry around with us or the laptops we use for our work all use some kind of battery. They are the reasons why we can carry these devices wherever we go without worrying about plugging them into a socket every time we wish to use them.
Even our cars and bikes use batteries. Regardless of what type of battery we use, whether lithium-ion batteries or nickel-metal hydride batteries, they all serve the same purpose: to store electrical energy. Every single battery has a voltage marking, also known as standard cell potentials, that tells us how much energy a battery can hold.
CALCULATING STANDARD CELL POTENTIALS
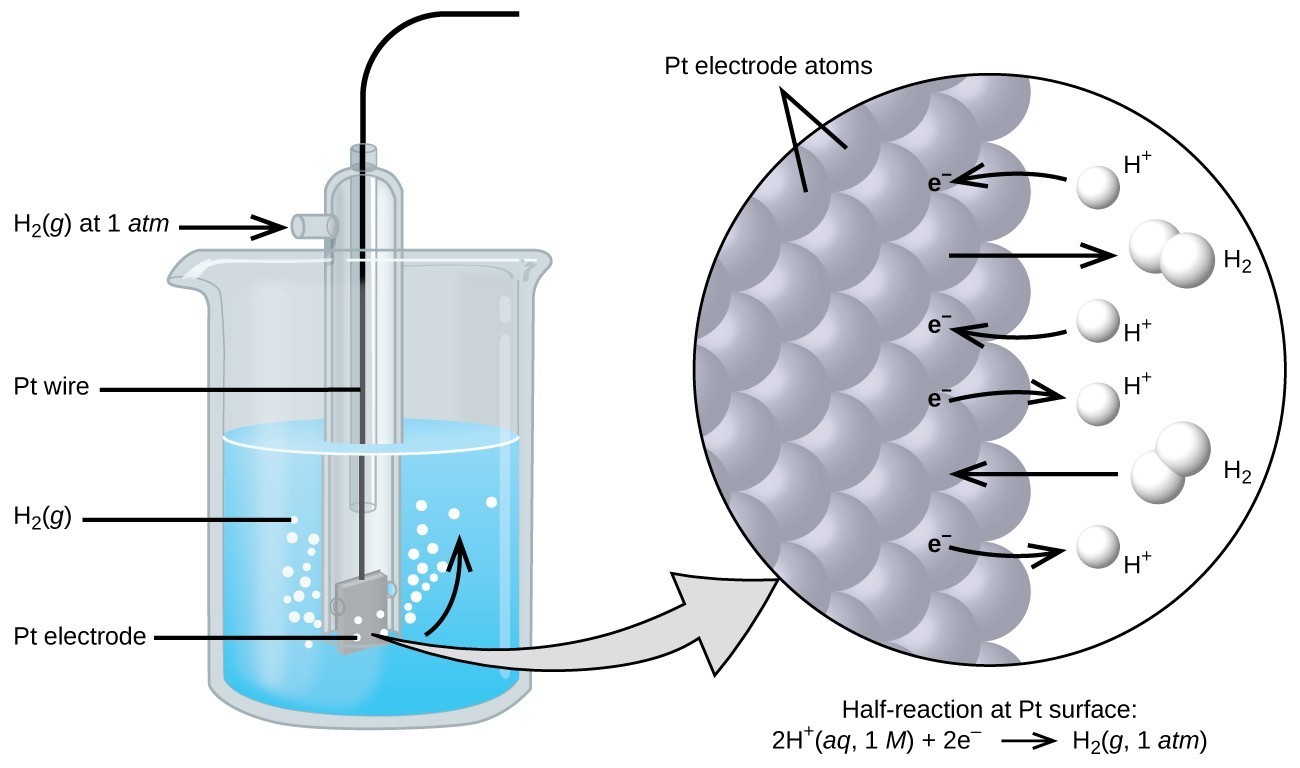
While determining the action voltage (pressure through an electrical circuit that pushes charged electrons) of a single electrode is difficult, we may give one a value of zero and use this as a baseline. The standard hydrogen electrode (SHE) is the electrode designated the zero value . The SHE comprises 1 atm hydrogen gas bubbling through a 1 M HCl solution at ambient temperature. The electrode is made of platinum, which itself is relatively inert.
Standard cell potentials (SRP) may be computed by subtracting the standard anode reduction potential from the standard cathode reduction potential.

The standard reduction potential of every element is already determined, and we can use this data while calculating the standard cell potential. The table below shows the SRP of different elements. There are many other uses of this data like finding out which element can act as an oxidizing agent or a reducing agent in a reaction.
OXIDIZING AND REDUCING AGENTS
There are several types of oxidizing agents and reducing agents. The standard reduction potentials may be thought of as a ranking of compounds based on their ability to oxidize and reduce. Compounds with high oxidation levels or high electronegativity that acquire electrons in the redox process are strong oxidizing agents.
The reactants in the SRP table below H+ are greater oxidants than H+, whereas those above it are weaker.
CONCLUSION:
- The standard cell potential is the voltage evaluated against a standard hydrogen electrode assuming standard circumstances.
- Any electrochemical cell must be made up of two half-cells to work.
- Standard cell potentials may be computed by subtracting the standard anode reduction potential from the standard cathode reduction potential.
FAQs:
1. How do you calculate the standard cell potential?
Subtract the standard reduction potential for the reaction at the anode out from the standard reduction potential for the electrochemical process at the cathode. It gives us the standard reduction potential of the cell. Since oxidation is the inverse of reduction, the negative sign is required.
2. What is electrode potential?
The potential of an electrode is defined as the potential of a unit in which the electrode in question serves as the cathode, and the standard hydrogen electrode serves as the anode. The anode is constantly oxidized, whereas the cathode is indeed reduced.
3. How do you calculate standard cell potential from Delta G?
The Gibbs free energy in a galvanic cell is linked to the potential by ΔG°cell = −nFE°cell.If E°cell > 0, then the reaction is feasible.
4. What does electrode potential depend on?
The oxidation potential is the propensity of an electrode to shed electrons, whereas the reduction potential is the capacity of an electrode to receive electrons. The potential of electrodes is determined by the concentration of metal ions and the temperature.
We hope you enjoyed studying this lesson and learned something cool about How to calculate standard cell potential?! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Calculating Standard Cell Potentials. https://www.ck12.org/c/chemistry/calculating-standard-cell-potentials/lesson/Calculating-Standard-Cell-Potentials-CHEM/. Accessed 27 Jan 2022.
- Oxidising and reducing agents. https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Comparing_Strengths_of_Oxidants_and_Reductants. Accessed 27 Jan 2022.
- Calculating Standard Cell Potentials. https://courses.lumenlearning.com/suny-albany-chemistry/chapter/standard-reduction-potentials/. Accessed 27 Jan 2022.