Electrolytic Cells Study Guide
INTRODUCTION
We always keep a special check over the battery percentage of our phones! We rely on rechargeable batteries to operate our smartphones, desktop computers, and other portable electrical gadgets. What do we do when the battery dies?
Batteries use a chemical process to produce electrical power, but this process slows down. You must invert the reaction to replenish the battery. This requires electric power, and you must charge the battery by plugging it in. The battery functions as an electrolytic cell while charging itself.
ELECTROLYTIC CELLS
To define electrolytic cells, we need first to understand electrolysis. Electrolysis is a method that entails passing an electric current through a liquid containing ions, causing the compounds inside to disintegrate. This is used to isolate the metal from metallic elements, segregate other chemical substances (such as water) and electroplate metals, and recharge batteries. A complete circuit is essential to maintain an electrolytic process; we need to be able to draw electricity from the cell continually.
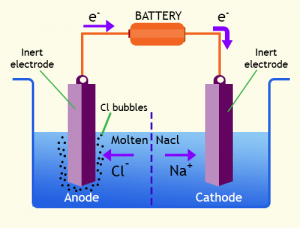
An electrolytic cell’s cathode and anode are connected to a power source. These two stable electrodes and a fluid electrolyte solution are found in every electrolytic cell. The electrolyte solution conducts electricity since dissolved ions can move freely in the solution.
WHAT HAPPENS INSIDE AN ELECTROLYTIC CELL?
Let’s have a look at the electrolytic cell diagram:
The battery initially offers an electrical energy source by forcing electrons onto the cathode, which makes it negatively charged. The anode is also positively charged because electrons are taken out of it. An oxidation-reduction reaction is triggered as a result of this. An oxidation reaction happens at the anode, generating electrons drawn to the positive anode. Simultaneously, a reduction process occurs at the cathode, which consumes the electrons accumulated on the cathode. These reactions can be written as :
Oxidation half of the reaction: A- –> e- A-Reduction half of the reaction: B e- –> B
The end outcome of each of these processes is that two ions become neutral atoms (A or B). This is achieved by the battery’s electrical energy, which transfers electrons from the anode to the cathode.
CONCLUSION
-
Running electric current through a conductive solution or molten salt causes a chemical reaction called electrolysis.
-
A cell made up of electrolytes and electrodes used to drive a non-spontaneous redox reaction is called an electrolytic cell.
-
The two reactions in an electrolytic cell are:
Oxidation A- --> e- A- Reduction B e- --> B
FAQs:
1. How do electrons flow in an electrolytic cell?
Electrons pass from the anode to the cathode through an external circuit in an electrolytic cell.
2. Why do electrolytic cells require a battery?
Electrolytic cells require an external electron source, such as a DC battery, because they involve non-spontaneous processes.
3. How does charge travel in an electrolytic cell?
The negatively charged electrons flow from cathode to anode in an electrolytic cell.
We hope you enjoyed studying this lesson and learned something cool about Electrolytic Cells! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Electrolysis: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/electrolysis. Accessed 24 Feb 2022.
- Electrolytic Cells: https://www.ck12.org/c/chemistry/electrolytic-cells/lesson/Electrolytic-Cells-CHEM/. Accessed 24 Feb 2022.