Atomic Orbitals Study Guide
Introduction
Ancient astrologers were successful in establishing that the Earth revolves around the Sun. Over the years, the science behind this hypothesis advanced, and we came to an understanding that planets revolve around a star in a definite orbit due to the strong gravitational pull of the star in its solar system.
Similarly, physicists, after years of hard work, established that every substance is made of atoms, and every atom is made of electrons, protons, and neutrons. Electrons revolve around a subatomic particle known as a nucleus.
The path of the revolving electron is termed an orbital. However, there’s a big difference between orbits and orbitals. In the case of planetary orbits, you can predict the path because trajectory and location are known. This is not the case with atomic orbitals.
In this guide, we will try to answer the question — what is an orbital in chemistry?
WHY IS IT IMPOSSIBLE TO DRAW ORBITS FOR ELECTRONS?
If we determine the position of an electron at a specific point in time and locate it again after that point, we’ll find that its position has changed; however, we’re unable to find the momentum of a said electron in either scenario and are therefore unable to determine its trajectory.
This phenomenon is summarized by Heisenberg’s uncertainty principle, which states that it is impossible to accurately define the position and momentum of an electron simultaneously, even in theory. As a result, we cannot draw a conclusive orbit for an electron around the nucleus in the same way we can for a planet revolving around a star.
We use a 3D map of an electron’s movements as they occur at different times to attempt to overcome this problem. With these maps, we find that 95% of the time an electron stays close to its nucleus in a spherical path. This path is what we call an orbital, and is to an electron what an orbit is to a planet.
WHAT ARE DIFFERENT ORBITALS?
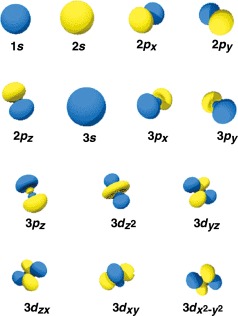
Every atomic orbital has a name depending on its proximity to the nucleus. In the case of hydrogen, which has one electron, the orbital is called 1s, meaning it is found at the first energy level of the atom closest to the nucleus. The 1 denotes the energy level, while the ‘s’ represents its shape, in this case spherical, like a chunky hollow ball with the nucleus at its center. Now, let’s take a look at this diagram:
In this diagram, the 2s orbital appears to be similar to the 1s orbital. However, in this case, the electrons revolve farther away from the nucleus. The number ‘2’ denotes that the orbital is at the second energy level.
Now, you may observe that the electron density is thicker around the nucleus. The reason behind this is that sometimes electrons spend some time near the nucleus to reduce the energy in s orbitals. Therefore, we can conclude that the electron energy decreases the closer they get to the nucleus. 3s, 4s, 5s, etc., are farther away from the nucleus.
P, D, AND F ORBITALS
There are other orbitals other than the s orbital. When we talk about the first energy level, the only orbital available for occupancy is the 1st orbital, which is denoted as 1s. But, at the second energy level, there are two orbitals available, 2s and 2p. A p orbital is shaped like two identical balloons.
Also, these identical balloons are tied to the nucleus. The orbital here denotes the highest chance of finding a particular electron. In a horizontal plane, the p orbitals are arranged at right angles to each other along the x, y, and z-axis.
These are denoted as px, py, and pz. P orbitals are available at higher energy levels too, which are denoted as 3p, 4p, 5p, and so on. As the energy level increases, the need for orbitals to accommodate electrons also arises.
Therefore, at the third energy level, there are a total of 5 d orbitals along with the s and p orbitals. This takes the total number of orbitals to 9. At the fourth energy level, we can find 7 f orbitals. This is in addition to the 4s, 4p, and 4d orbitals. Therefore, the total number of orbitals comes out to be 16.
WHAT ARE QUANTUM NUMBERS?
Quantum numbers are a set of numbers used to describe the electron’s position and energy inside an atom. Principal, azimuthal, magnetic, and spin quantum numbers are the four types of quantum numbers.
- Principal quantum number (n).
- Orbital angular momentum quantum number (or azimuthal quantum number) (l).
- Magnetic quantum number (ml).
- The electron spin quantum number (ms).
AUFBAU PRINCIPLE
The Aufbau principle shows how electrons are filled in an atom’s atomic orbitals when it is in its ground state. According to this theory, electrons are filled into atomic orbitals in the sequence of increasing orbital energy levels. According to the Aufbau principle, the lowest energy atomic orbitals are occupied first, followed by the higher energy levels.
PAULI EXCLUSION PRINCIPLE
The Pauli Exclusion principle asserts that no two electrons in a single atom will have the same set of quantum numbers (n, l, ml, and ms). To put it another way, each electron should have or be in its own distinct state (singlet state). The Pauli Exclusion principle is based on two main principles:
- Only two electrons can be in the same orbital at the same time.
- The spins of the two electrons in the same orbital must be opposing or they must be antiparallel.
HUND’S RULE
According to Hund’s rule:
Before any orbital in a sublevel is double occupied, it is singly occupied.In singly occupied orbitals, all electrons have the same spin (to maximize total spin).
SUMMARY
- Atomic orbitals are the path in which an electron revolves around the nucleus.
- It is impossible to plot the route of the electron because of Heisenberg’s uncertainty principle.
- The hydrogen atom has just one orbital known as 1s. 1s is the first energy level, which is closest to the energy of the nucleus.
- At the second energy level, the electron moves farther away from the nucleus and resides in a p-shaped orbital, referred to as 2p.
- At the third and fourth energy level, d and f orbitals come into play, and the total number of orbitals become 16.
FAQ’s
1. What is an orbital in chemistry?
Atomic orbitals are the path in which the electron revolves around the nucleus.
2. What are the four types of orbitals?
The four orbitals are mainly categorized as s,p,d, and f, depending on the energy level of the electrons.
We hope you enjoyed studying this lesson and learned something cool about Atomic Orbitals! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
REFERENCES:
- Atomic Orbitals: https://chem.libretexts.org/. Accessed 28 Feb 2022.
- Orbital: https://www.ck12.org/c/chemistry/orbital/lesson/Orbitals-CHEM/ Accessed 28 Feb 2022.