Le Châtelier’s Principle and the Equilibrium Constant Study Guide
INTRODUCTION
The working of a seesaw is no big surprise for anyone. But the physical and chemical facts that can be observed from it may be much deeper than you can imagine. Kids of the same weight positioned at equal distances from the seesaw’s center will counterbalance each other. In the same situation, children of different weights would not balance. In simple words, the smaller child would go upwards, and the bigger kid would go downwards. We can understand this phenomenon with the help of Le Châtelier’s Principle.
LE CHATELIER’S PRINCIPLES AND THE SEESAW
The equilibrium law, commonly known as Le Chatelier’s principles, is used to forecast the impacts of different systems in an equilibrium state (such as the change in temperature or pressure). Henry Louis Le Chatelier, a French chemist, was the inspiration for the theory.
When applying Le Châtelier’s principle, it’s helpful to think of the equilibrium as a see-saw. The equilibrium signifies the see- saw’s balancing point. Consider how a see-saw works: anything on the same part of the balance point goes in the same way, while everything on the opposite end of the balance spot swings in the reverse direction.
According to Le Chatelier, equilibrium modifies forward and backward reactions to tolerate changes altering the equilibrium circumstances. When equilibrium-affecting parameters such as concentration, pressure, temperature, and inert gasses are modified, the equilibrium will move towards the direction where the effects of these alterations are neutralized.
Nevertheless, because an equilibrium can only exist in a closed system, there is no use in saying that you can use Le Châtelier’s principle until you briefly open up the system to make a change. When you close the system again, it will return to equilibrium in the manner indicated by le Châtelier’s principle.
THE EQUILIBRIUM CONSTANT
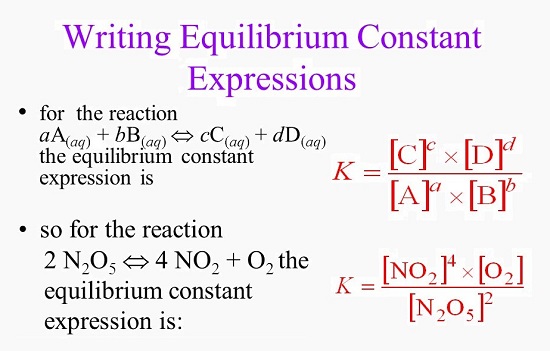
The equilibrium constant describes the connection between a reaction’s products and reactants when it is at equilibrium with regard to a given unit. We can create an equilibrium equation for a system in chemical equilibrium that enables us to predict the impact of changes in the system.
It’s important to remember that the equilibrium constant is just a constant. Whatever adjustments are made to the ratios of any reaction components, the net result must be the same as the initial constant.
CONCLUSION
- The equilibrium law, commonly known as Le Chatelier’s principles, is used to forecast the impacts of different systems in an equilibrium state.
- According to Le Chatelier, equilibrium modifies forward and backward reactions to tolerate changes altering the equilibrium circumstances.
- The equilibrium constant describes the connection between a reaction’s products and reactants when it is at equilibrium.
FAQs:
1. How do you explain Le Chatelier’s principle?
According to Le Chatelier, equilibrium modifies forward and backward reactions to tolerate changes altering the equilibrium circumstances.
2. How does Le Chatelier’s principle apply to real life?
Everyday tasks like drying clothes are also examples of Le Chatelier’s principle and chemical equilibrium in real life. On a windy day, the water vapors are carried away faster, now to establish an equilibrium, the water from the clothes starts drying, hence drying the clothes faster.
3. What is Le Chatelier’s principle used to predict?
The principle of Le Chatelier can be used to forecast how a system would behave in response to variations in pressure, temperature, or concentration.
We hope you enjoyed studying this lesson and learned something cool about Le Châtelier’s Principle and the Equilibrium Constant! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Watch the Shift. https://www.ck12.org/c/chemistry/le-ch%C3%A2teliers-principle-and-the-equilibrium-constant/rwa/Watch-the-Shift/. Accessed 17 Feb 2022.
- Le Chatelier’s principle. https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/le-chateliers-principle/11161/. Accessed 17 Feb 2022.