Energy In Chemistry Study Guide
Introduction
- Every organism uses different types of energy to power the biological processes that help them grow and survive.
- Energy is nothing but a property of an object that can easily be passed from one thing to another either directly or by converting it into different forms.
- Though it can be transferred and changed, energy can neither be created nor destroyed.
WHAT IS ENERGY
- Energy, derived from the Greek word “energia” for an operation or activity, is the capacity to do work.
- It can be transferred from one body to another, and exists in multiple forms, such as kinetic energy, potential energy, chemical energy, electrical energy, and more.
- The large extent of energy for life on earth is derived from the sun in the form of solar energy.
- The SI unit of energy is the Joule (J).
- A joule is said to be consumed when one newton of force is applied to one meter of distance.
DIFFERENT TYPES OF ENERGY
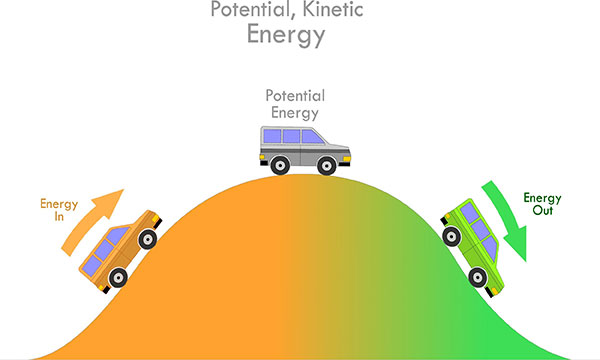
1. KINETIC ENERGY
The energy possessed by any moving object by virtue of its motion is called kinetic energy, and can be calculated with the following equation.
KE = ½mv2,
- Where m is the mass
- and v is the velocity of the moving object.
Examples of kinetic energy include things like blowing wind, birds flying, or a person riding a bike. Source
Source
2. POTENTIAL ENERGY
The moving object stores the energy due to its position relative to other objects, which is known as potential energy.
PE = mxgxh,
- Where, m is mass,
- g is the acceleration due to gravity,
- and h is height.
3. MECHANICAL ENERGY
It is generally the sum of kinetic and potential energy.
M.E = m x g x h plus ½mv2
SUMMARY
- Energy is the most fundamental concept of chemistry.
- Basics of energy can neither be created nor destroyed.
- It is the capacity to do work with the standard units as joule.
- It has two main types of energy kinetic and potential energy, collectively as mechanical energy.
- Some other types are chemical, thermal, electrical, nuclear, motion, etc.
FAQ’s
1. What is energy in science?
Energy is defined as the ability to do work. Energy can change its form but can neither be created nor destroyed.
2. Why is energy important in chemistry?
Energy is important in chemistry because it enables industrial chemical reactions to produce raw materials. And these raw materials are further used to make useful final goods like cars, bridges, etc.
3. What are the main uses of energy?
Uses of energy:
- Generating electricity
- Drive a bicycle
- Heating and cooling of bulbs
- Moving freights
- Various other commercials, residential and transport facilities.
4. Why is the energy needed?
Energy is the basic need of a living organism. It is used to generate heat and light and produce several humans made things. Energy is used to improve and save various living organisms.
We hope you enjoyed studying this lesson and learned something cool about interpreting Energy In Chemistry! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun!😎
REFERENCES
- What is Energy? https://www.eia.gov/energyexplained/what-is-energy/. Accessed 28 Feb 2022.
- The Nature and Types of Energy: https://chem.libretexts.org/Courses/Eastern_Wyoming_College/EWC%3A_CHEM_1030_-_General_Chemistry_II_(Budhi)/06%3A_Thermochemistry/6.02%3A_The_Nature_and_Types_of_Energy. Accessed 28 Feb 2022.
- Types of Energy: https://courses.lumenlearning.com/introchem/chapter/types-of-energy/. Accessed 28 Feb 2022.
- Forms of Energy: https://www.vedantu.com/physics/forms-of-energy. Accessed 28 Feb 2022.
- Forms of Energy: https://www.eia.gov/energyexplained/what-is-energy/forms-of-energy.php#:~:text=Chemical%20energy%20is%20energy%20stored,gasoline%20in%20a%20car’s%20engine.Accessed 28 Feb 2022.