Ionic Bonding Study Guide
Introduction
There are several different types of bonds and forces that are known to bind different molecules together. The two most common chemical bonds we come across are covalent and ionic bonding, where atoms easily transfer their electrons to each other.
Well, what exactly is ionic bonding? Keep reading to find out!
WHAT IS TYLER DEWITT IONIC BONDING?
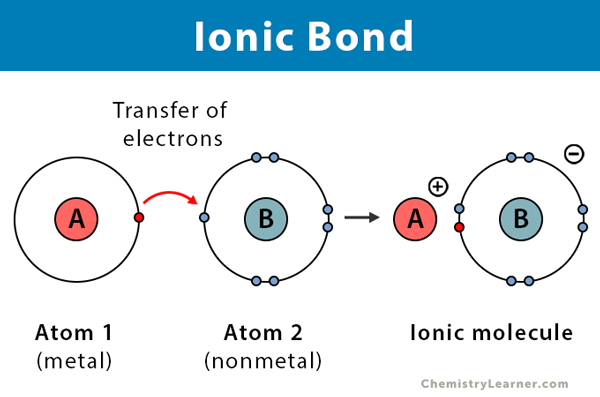
- Ionic bonding can be defined as the electrostatic force of attraction which tightly holds the two very oppositely charged ions together.
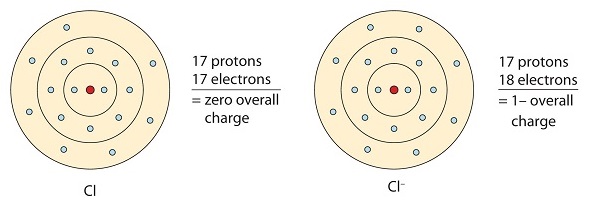
- A chemical bond is formed between two known atoms through the complete transfer of one or even more electrons from one significant atom to another; this results in the atoms attaining the nearest inert gas configuration.
Mainly, there are three ways by which atoms could attain stability:
-
Sharing of electrons, that results in creating a covalent bond between the atoms.
-
Sharing of electrons by one atom only and not by both atoms.
-
Transfer of electrons and completing the octet configuration. The bond that is then formed is termed an electrovalent bond or ionic bond.
-
This bond signifies one atom losing electrons while the other atom gains electrons in its outermost shell.
-
Electrovalent bonds result in the formation of positive and negative ions.
-
Electrovalent bonds are only observed between metals and non-metals.
-
They cannot form between two nonmetals.
-
More simply, through electrovalent bonding, a certain number of electrons from one atom move towards a different atom so that both can attain a stable inert gas configuration.
WHAT ARE THE MAJOR PROPERTIES OF IONIC BONDING?
You would witness the following properties in ionic bonding because of a strong force that tightly holds the cations and anions in ionic bonding molecules.
- The ionic bonds are considered to be the strongest among all other chemical bonds.
- Under the proper medium, these bonds are the most reactive as they have charge separation.
- Ionic bonding molecules have high boiling and melting points.
- The molecules that showcase ionic bonding, in their aqueous solution or even in their molten state, are good electricity conductors. This phenomenon happens as the ions are charge carriers.
WHAT IS TYLER DEWITT COVALENT BONDING?
- Covalent bonding can be termed as sharing of electrons between atoms.
- This type of bonding happens between two atoms of the same elements or atoms of elements that are very close to each other in the periodic table.
- Covalent bonding can be primarily observed between nonmetals; however, in some cases, it can also be observed between metals and nonmetals.
- The Tyler Dewitt covalent bond occurs when the difference between the electronegativities of two atoms is way too small for an electron transfer to occur.
SUMMARY
- Ionic bonding is defined as the electrostatic force of attraction that holds the two oppositely charged ions together strongly.
- Electrovalent bonds result in negative and positive ions.
- Covalent bonding can be termed as sharing of electrons between atoms.
FAQs
1. What is a covalent bond Tyler Dewitt?
A covalent bond can be defined as an interatomic linkage that results in the sharing of an electron pair between two atoms.
2. How do you explain ionic bonding?
Ionic bonding can be defined as the electrostatic force of attraction which tightly holds the two very oppositely charged ions together.
We hope you enjoyed studying this lesson and learned something cool about Ionic Bonding! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
Sources
- Ionic Bond: https://byjus.com/chemistry/ionic-bond-or-electrovalent-bond/.Accessed 10th March 2022.
- Ionic and Covalent Bonds: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Ionic_and_Covalent_Bonds.Accessed 10th March 2022.
- The Ionic Bond: https://courses.lumenlearning.com/boundless-chemistry/chapter/the-ionic-bond/.Accessed 10th March 2022.