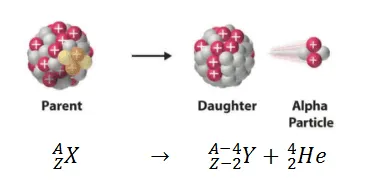Types of Radioactive Decay Study Guide
INTRODUCTION
Do you know that Marie Curie, who was awarded the Nobel Prize twice, died because of the discovery she made? And the same discovery is now showing its wonder in the field of medicine. Yes, you read it right.
Madame Curie died because of aplastic anemia caused by exposure to radiation. But what kind of radiation? Let’s find out by discussing different types of radioactive decay in this article.
WHAT IS RADIOACTIVE DECAY?
Everything in nature wants to be more stable, and so is it too with an atom. When there is an imbalance of protons and neutrons in a nucleus, it disintegrates and gives out radiation by a process called radioactive decay. The atom can disintegrate in various ways, such as alpha, beta and gamma decay.
It is to be noted that the sum of atomic number and mass number of reactants and products remains the same in all of the following processes.
ALPHA DECAY(𝞪):
When a nucleus breaks up into a helium nucleus (alpha particle) and an atom called a daughter nucleus. The helium nucleus has 2 protons and 2 neutrons and is positively charged. The daughter nucleus has atomic number 2 less and mass number 4 less than the parent nucleus.
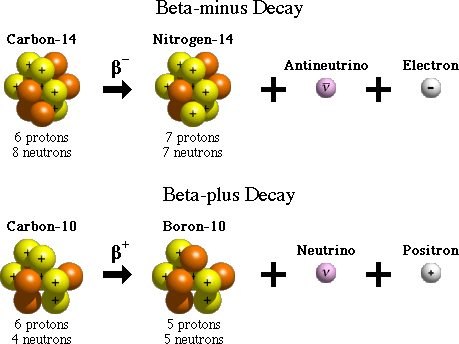
BETA DECAY(𝛃):
Beta-decay is of two types: 𝛃- and 𝛃.
The easiest way to remember the types is that 𝛃- gives out a negatively charged particle and 𝛃 gives a positively charged one.
The emission of an electron is 𝛃- decay, and it occurs when the neutron to proton ratio of a nucleus is high.
It is the conversion of a neutron to a proton and electron accompanied by the release of some energy. There is no change in mass number, but the atomic number increases by 1 for the daughter nucleus.
𝛃 OR POSITRON DECAY:
This process is exactly the opposite of 𝛃- decay, as this occurs when the neutron to proton ratio in an atom is low. In this process, a proton is converted to neutron and positron. The positron can be thought of as a twin of an electron but with an opposite charge. The mass number of the daughter nucleus decreases by 1 in this process.
ELECTRON CAPTURE:
In this process, the inner electron of an atom is consumed by a neutral nucleus. Since one electron is consumed by the nucleus, the atomic number decreases by 1, but the mass number doesn’t change. Also, this process is similar to 𝛃 decay.
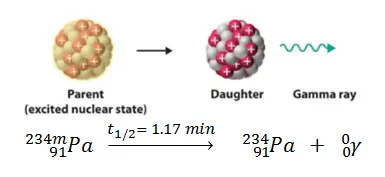
GAMMA EMISSION (Ɣ):
Gamma emission is neither accompanied by a change in mass number nor in atomic number. Then what is changing? The energy.
The excited nucleus, after emitting alpha or beta particles, on its return to a lower energy state, emits gamma radiation.
CONCLUSION:
- An atom with an imbalanced number of neutrons and protons breaks and gives out radiation in the form of some particles.
- Depending on the particle emitted and change in mass and atomic number, the decay is called alpha, beta and gamma decay.
FAQS:
1. Why in beta decay, the mass number of the daughter nucleus doesn’t change?
During beta decay, an electron is ejected, which has no mass or negligible mass. Thus, the mass number doesn’t change.
2. Can gamma decay occur independently?
No, gamma decay occurs only after a nucleus undergoes alpha or beta decay.
3. What is alpha decay?
When an atomic nucleus emits an alpha particle, it results in a decrease in the atomic and mass number of the daughter nucleus.
We hope you enjoyed studying this lesson and learned something cool about the Types of radioactive decay! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Radioactive Decay Types: https://www.khanacademy.org/science/in-in-class-12th-physics-india/nuclei/in-in-nuclear-physics/a/radioactive-decay-types-article. Accessed 25 Feb 2022.
- Gamma Decay: https://www.ck12.org/chemistry/gamma-decay/lesson/Gamma-Decay-MS-PS/. Accessed 25 Feb 2022.
- Radioactive decay: https://en.wikipedia.org/wiki/Radioactive_decay. 25 Feb 2022.