Alkyl Halides Study Guide
INTRODUCTION
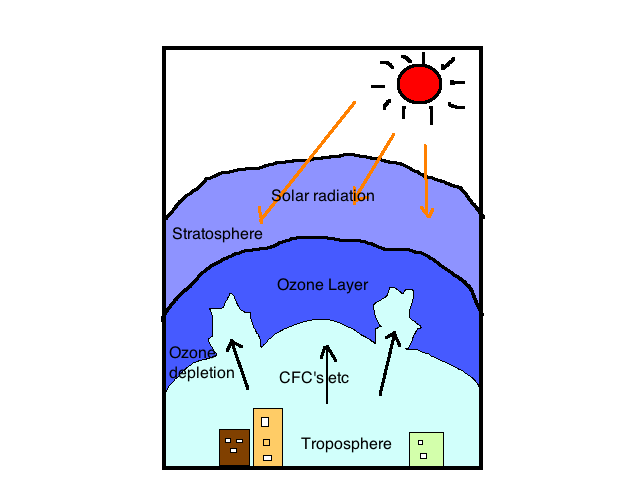
Have you ever wondered what keeps your precious chocolate ice cream from turning into a chocolate shake? Obviously, the answer is a freezer, but what is the scientific way to answer it? Refrigerators in our kitchens use refrigerants such as chlorofluorocarbons (an alkyl halide), which helps freeze our ice creams.
However, the impacts of ozone-depleting substances (ODS) on the stratospheric ozone layer have been a source of worry. In the 1970s, various countries, notably the United States, banned the use of chlorofluorocarbons (CFCs) as aerosol sprays. These threatening yet useful gases here are an eminent example of what we are going to study in this article, the alkyl halides in organic chemistry.
WHAT ARE ALKYL HALIDES?
An alkyl halide is an organic compound in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine, or iodine)
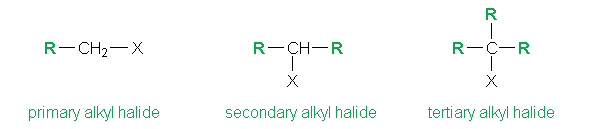
In the above example, the letter R stands for alkyl group and the X for a halogen atom. We utilize the generalized formula R-X to describe an alkyl halide with 3 further categories on the basis of whether the halogen atom is bonded to a primary, secondary or tertiary carbon atom. (The primary carbon atom is the carbon which is attached to only one other carbon atom. Similarly for secondary and tertiary).
NOMENCLATURE OF HALOALKANES
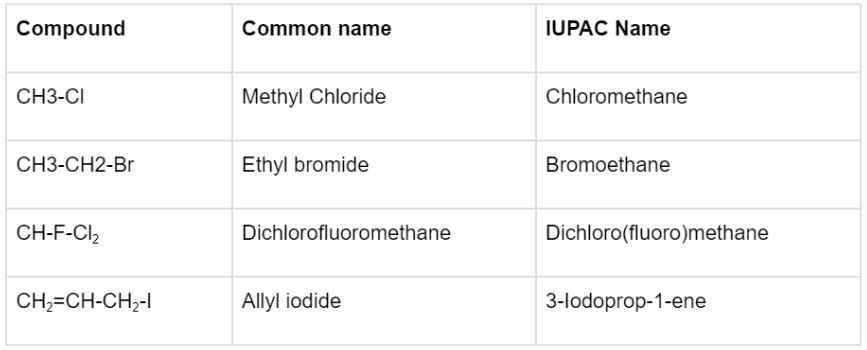
The question of how to name alkyl halides (also known as haloalkanes) can be answered in 2 ways: the common name and the IUPAC name. The simple steps to do so are listed below by taking CH3CH2CH2Br as an example:
- Find the longest carbon chain. Here, the propyl group is the longest carbon chain.
- Position the longest carbon chain so that the halogen(s) is/are linked to the carbon atom(s) with the lowest number (s). Here, we will position the carbon next to the Bromine as the first carbon.
- When identical halogen atoms are linked to a carbon atom, multiple halogen atoms are labelled with Greek numerical prefixes such as di, tri, and tetra. The above example has only one bromine so no such prefix would be used.
- When several types of halogens are attached, they are designated alphabetically. In case the above example had a Chlorine linked to it, then the order of naming would have been Bromine followed by Chlorine.
- The halogen atom’s position is specified by placing the halogen’s location and title just before the parent hydrocarbon’s name. The position of the halogen here is 1-Bromo.
- The example’s final IUPAC name is 1-bromopropane.
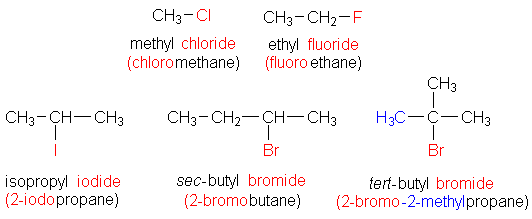
Some of the examples of alkyl halides are listed below:
Note:Because there is just one carbon atom in the structure based on methane, there is no need to employ a number. The chloro is listed first alphabetically in the third example.
Classification Of Alkyl Halide
Alkyl halides may be divided into three major categories: primary (1°), secondary (2°), and tertiary (3°).
- Primary or (1°)alkyl halides have a halogen atom connected to a carbon atom that is only bonded to one other carbon atom next to it, such as ethyl chloride CH3CH2Cl.
- Secondary or (2°) alkyl halides, such as isopropyl chloride CH3CH(Cl)CH3, have a halogen atom connected to a carbon atom that is bonded to two additional carbon atoms.
- Tertiary or (3°) alkyl halides, such as tert butyl chloride (CH3)3CCl, have a halogen atom connected to a carbon atom that is bonded to three additional carbon atoms.
Alkyl halides are hence alkanes with a halo atom acting as a functional group. They are divided into three groups: elementary (1°), secondary (2°), and tertiary (3°).
Alkyl Halide Properties
-
State: The states of alkyl halides include solid, liquid, and gaseous. As lower member gases, they can be found in methyl chloride, ethyl bromide, ethyl chloride, etc. Up to C18, the higher members exist as liquids. Greater members, such as those above C18, are present in solid alkyl halides.
-
Odour: All alkyl halides are largely odourless, however up to C18, alkyl halides have a nice, sweet aroma.
-
Color: Alkyl halides in their purest form have no colour. When kept for prolonged periods of time, iodoalkanes and bromoalkanes take on bright colours.
-
Boiling point: Alkyl halides have high boiling points in comparison to other halides. Because of their high polarity and robust dipole interaction, this is the case. Alkyl halide compounds and London forces interact more intensely than other molecules (van der Waals force).
Chemical Reactions
Substitution Reaction
Alkyl halides undergo substitution reactions where an alkyl halide or halogen is exchanged with another molecule, producing a saturated hydrocarbon as a byproduct.
For instance, CH3Cl + OH- → CH3OH + Cl-
Elimination Reaction
Dehydrohalogenation is another name for an elimination reaction. In this reaction, the halogen and a proton are taken out to produce an alkene. Dihaloalkanes can also be changed into alkynes using the same procedure.
For instance, CH3CH2Cl + RO- → CH3CH2OR + Cl-
Hydrolysis
Water breaks a bond in this process. This demonstrates the nucleophilic character of alkyl halides. Ethanol is produced when bromoethane is hydrolyzed.
For instance, CH3Br+OH- →CH3OH+Br-
USES OF ALKYL HALIDES
Alkyl halides are an integral part of organic chemistry and are widely used throughout, some of them being:
- In chemistry, short-chain haloalkanes are frequently utilized as hydrophobic solvents.
- Chlorofluorocarbons (CFCs) were widely utilized as coolants and propellants. Halon 1211 and Halon 1301, as well as others, are used in fire extinguishers.
- Initially, haloforms (an alkyl halide) were used as a sedative.
- Inhalation anesthetic halothane (2-bromo-2-chloro-1,1,1-trifluoroethane) has been used in specific instances.
CONCLUSION
- An alkyl halide is an organic compound in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine, or iodine)
- A certain set of IUPAC norms have to be followed to name these alkyl halides.
- Alkyl halides are an integral part of organic chemistry and are used as a refrigerant, hydrophobic solvents, chlorofluorocarbons (CFCs), fire extinguishers, etc.
FAQs
1. What is the general formula of Haloalkanes?
A: The compounds with the generic formula “RX,” where R is an alkyl or a substituted alkyl group, and X is a halogen, are called haloalkanes or alkyl halides (F, Cl, Br, I).
2. What is the general formula of aryl halides?
A: Aryl halides have the generic formula ArX, where Ar is phenyl, branched phenyl, or aryl groups.
3. What is alkyl halide?
A: Alkyl halides, commonly known as haloalkanes or halogenoalkanes, are chemical compounds made up of one or more halogens.
4. What is alkyl halide and aryl halide?
A: Alkyl halides, commonly known as haloalkanes or halogenoalkanes, are chemical compounds made up of one or more halogens. Aryl halides are compounds with a halogen directly bonded to an aromatic ring carbon.
We hope you enjoyed studying this lesson and learned something cool about Alkyl Halides General Formula! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
]]>