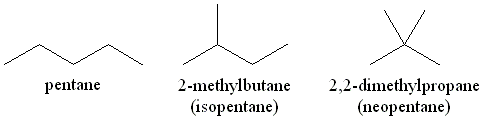Branched Alkanes Study Guide
INTRODUCTION
Who does not wish to travel back in time to trace their great-great-grandparents. Unfortunately, none of us is Marty McFly (from Back to the Future franchise), and the only logical way to unveil your family history would be by tracing a family tree. The details of this tree have to be looked into carefully so that you may not end up declaring Hitler to be your Uncle. 🤭You need to take similar care while studying the branching of alkanes.
BRANCHED ALKANES
A saturated hydrocarbon is known as an alkane, and it can be straight-chained, Linear, branched, or even cyclic.
An alkane with alkyl groups linked to its main carbon chain is a branched-chain alkane or branched alkane. Branched alkanes include carbon and hydrogen (C and H) atoms, and carbon atoms are only coupled to certain other carbons by single bonds. Still, the molecules have branches like methyl, ethyl, etc., and are thus not simple.
ALKANE EXAMPLE
Starting with pentane as an alkane example, there are two alternative structures for this structure that are not straight-chain and are rather branched. These alternatives are termed structural isomers. They are multiple structures that can be formed with molecules having the same molecular formula.
For instance, here 2 -methylbutane and 2,2 – dimethylpropane or neopentane are the structural isomers of the given pentane. 2-methylbutane here is an easy-branched alkane example. However, the names of these structures are not something you can cram. These names are coined according to IUPAC rules. It’s important to learn the rules for naming branched alkanes to create a universal standard for identifying chemicals to make communication easier.
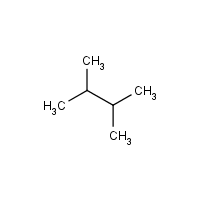
Let’s try understanding the naming of these branched alkanes with this isomer of hexane –
- First, find the longest chain of carbon in the structure that is continuous. In this case, it will be the chain linking carbon 1 and carbon 4.
- Begin at the end, which will result in the minimum possible numbers for the carbon atoms wherein the branches arise. As here, both the carbons have identical positions to start either way.
- The attached atoms are called substituents. These are called alkyl groups. The prefixes here are the same, but the suffix has to be ‘yl.’ In the current structure, we have 2-methyl and 3-methyl.
- A prefix has to be used in case 2 or more similar substituents are present. In the example, we will have 2,3-dimethyl.
- Different substituents have to be placed alphabetically. For instance, 2-ethyl comes before 4-methyl.
- At last, commas have to be used to separate numbers and hyphens between the number and the name of the substituent.
The IUPAC name of the branched alkane is 2,3-dimethylbutane.
CONCLUSION:
- Branched alkanes include carbon and hydrogen (C and H) atoms.
- An alkane with alkyl groups linked to its main carbon chain is a branched-chain alkane or branched alkane.
- The names of branched alkanes are established according to IUPAC.
FAQs:
1. How do you name branched alkanes?
- Compute the number of carbon atoms in the longest chain.
- Beginning with the endpoint closest to a branch, count the carbons in the chain.
- Count how many carbons are in each branch.
- Look for repetitive alkyl groups.
- In alphabetical sequence, but the labels of the substituent groups in front of the parent chain’s name.
2. What is straight and branched-chain alkane?
A saturated hydrocarbon is known as an alkane, and it can be straight-chained, Linear, branched, or even cyclic.
3. What is branched and unbranched?
An alkane with linked alkyl groups on its parent chain is called branching, where the ones with no link are called unbranched.
We hope you enjoyed studying this lesson and learned something cool about Branched Alkanes! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Branched Alkanes. https://www.ck12.org/c/chemistry/branched-alkanes/lesson/Branched-Alkanes-CHEM/. Accessed 1 Feb 2022.
- Alkanes. https://www.angelo.edu/faculty/kboudrea/molecule_gallery/01_alkanes/00_alkanes.htm. Accessed 1 Feb 2022.
- How to Name Branched Alkanes in Chemistry. https://www.dummies.com/article/academics-the-arts/science/chemistry/how-to-name-branched-alkanes-in-chemistry-143029. Accessed 1 Feb 2022.