Ethers Study Guide
INTRODUCTION
We have heard of medical doctors who administer anaesthesia to help manage pain during surgery. The main ingredient in anaesthesia is diethyl ether. Yes, you read that right! It’s diethyl ether, which belongs to the family of ethers. The discovery of ether allowed physicians to use more refined techniques of surgery and medications. After the invention of safer, more effective inhalation anaesthetics, the usage of ether and chloroform fell, and they are no longer used in surgery.
WHAT IS ETHER?
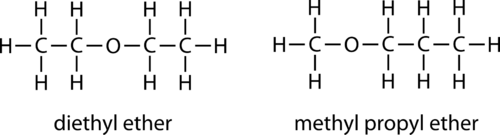
Ethers are a group of organic compounds in which an oxygen atom is bonded with an alkyl or aryl group. It is represented by the general Ethers formula:
The R in this formula represents the hydrocarbon group. It can be the same as R’ or different.
NOMENCLATURE OF ETHERS:
There are a set of rules to follow before naming an ether.
- Firstly, you must select the longest carbon chain as a base, giving it its base name.
- Secondly, you should change the name of your hydrocarbon group ending with ‘yl’ to ‘oxy.’ Now, for example, butyl becomes butoxy and methyl becomes methoxy.
- Now the second name comes in front of the base name.
PROPERTIES AND USES OF ETHERS:
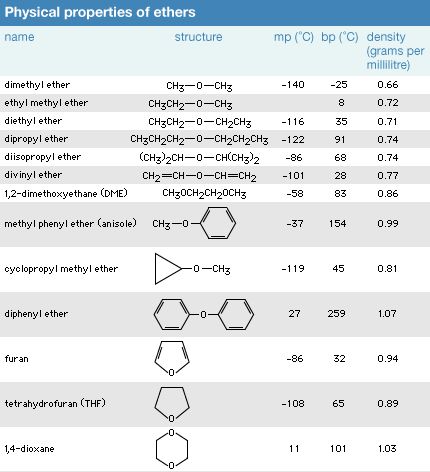
- Ethers are colorless, pleasant-smelling liquids at room temperature.
- Ethers have lower boiling temperatures and are less dense than alcohols.
- They are also less soluble in water.
- Because they are generally inert, they can be used as solvents for fats, oils, waxes, and fragrances.
- Dipole moments of two C-O atoms do not cancel each other, which is why they possess a small net dipole.
- Ethers are also useful in medicine and pharmaceuticals, particularly as anesthetics.
- A methyl ether of morphine is codeine, a powerful pain reliever.
- Ethyl ether is a versatile solvent that may be used for the extraction process and several chemical processes.
- In cold weather, it’s also utilized as a volatile starter liquid for diesel and gasoline engines.
CONCLUSION
- Ethers are a group of organic compounds in which an oxygen atom is bonded with two alkyl or aryl groups.
- The general Ethers formula is R-O-R’. Here R represents the hydrocarbon group, and R’ can be the same or different hydrocarbon group.
FAQs:
1. What is the general formula of ethers?
The general representation of ether is R-O-R’, where R represents hydrocarbon group and R’ can be the same or different hydrocarbon group.
2. What is the formula of ether and ester?
- The general formula of ether is R-O-R’
- The general formula of the ester is R-COO-R’
Here, R represents the hydrocarbon group, and R’ can be the same or different hydrocarbon group.
3. What are ethers’ examples?
Some examples of others are:
- Dimethyl ether, represented as CH₃ – O – CH₃
- Ethyl methyl ether, represented as CH₃ – O – CH₂ – CH₃
4. How do you write ethers?
There are rules to follow before naming an ether in chemistry. The rules are:
- Firstly, you must select the longest carbon chain as a base, giving it its base name.
- Secondly, you should change the name of your hydrocarbon group ending with ‘yl’ to ‘oxy.’ Now, for example, butyl becomes butoxy and methyl becomes methoxy.
- Now the second name comes in front of the base name.
We hope you enjoyed studying this lesson and learned something cool about the Ethers formula! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Ethers. https://byjus.com/jee/ethers/, Accessed on 26th Jan 2022.
- Ethers. https://www.ck12.org/c/chemistry/ethers/lesson/Ethers-CHEM/, Accessed on 26th Jan 2022.