Periodic Trends: Ionization Energy Study Guide
INTRODUCTION
We all know how difficult it is to teach in a preschool, don’t we? You want the excited young students to stay together and maintain uniformity, but this stability can barely be achieved. A student may carelessly wander off to other strong, attractive forces of a bright crayon, a fluffy toy, or their parents. The worst is that a student may be scared for some reason and may run off screaming. Now, a student’s choice to act individually or with the class depends on the balance.
Similarly, the electrons and protons in an atom are always in conflict. The ease with which electrons can be withdrawn from an atom affects the atom’s reactivity, and this quantity can be measured and used to make accurate predictions about atom behavior.
DESCRIBE IONIZATION ENERGY
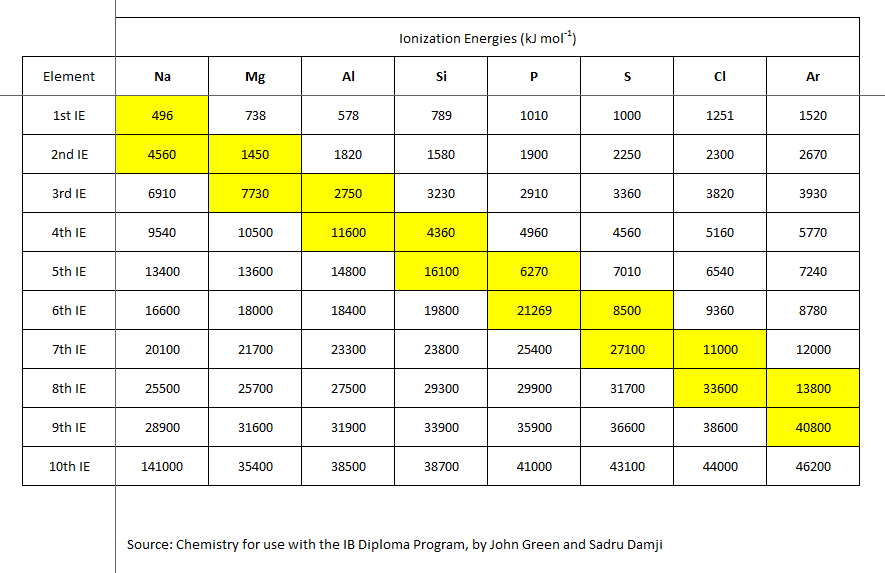
The energy required to remove an electron from a certain atom is ionization energy. It is expressed in kJ/mol, an energy unit similar to calories. The table below shows some of the ionization energies linked with several elements. Any atom’s outermost valence electrons will have lower ionization energies than the inner-shell electrons. As more electrons add around a nucleus, the inner shell electrons shield the outer electrons from the nucleus. This is termed electron shielding.
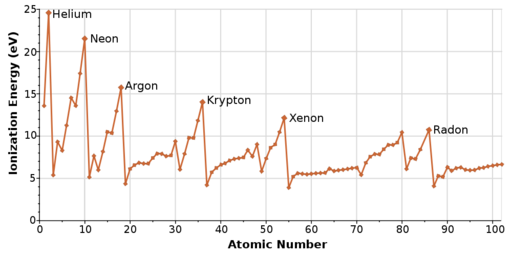
We may see the following trend if we describe the trends in first ionization energy vs. atomic number for the major group elements on a graph.
PERIODIC IONIZATION ENERGY TREND
The ionization energy of electrons in different atomic or molecular orbitals is variable. The xth ionization energy is the energy needed to eliminate the nth electron after the first x-1 electrons have been withdrawn. It measures an atom’s or ion’s tendency to relinquish an electron or the intensity of the electron binding.
The more ionization energy there is, the harder it is to remove an electron. The ionization energy of an element can be used to determine its reactivity. The low one’s elements tend to be reducing agents.
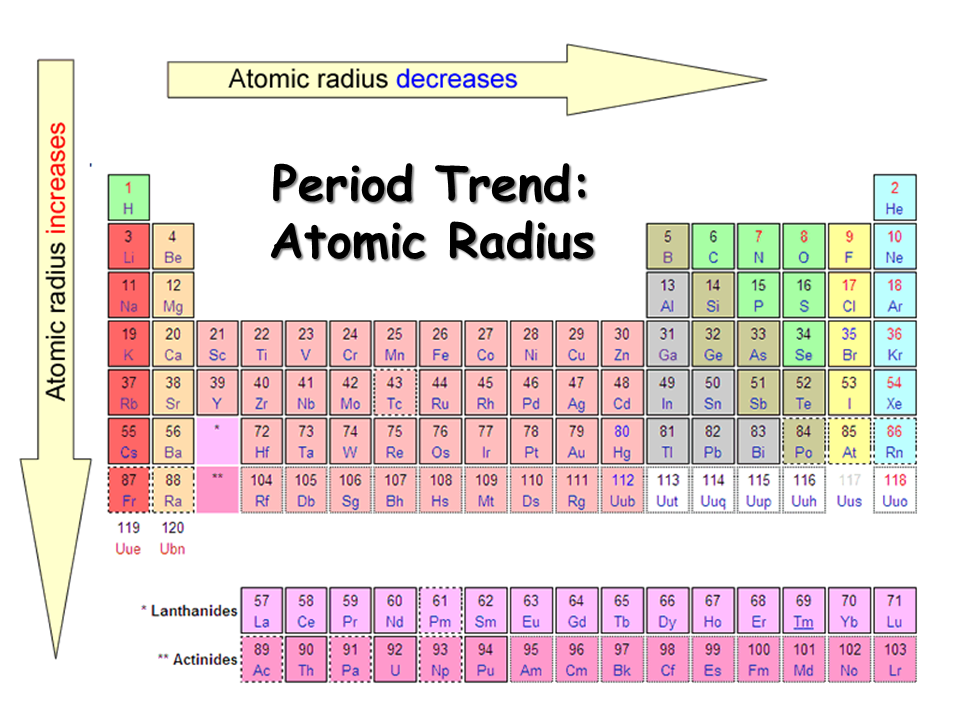
As you move across a period, the first ionization energy increases. As the atomic size reduces, it becomes more difficult to eliminate an electron nearer to a more central nucleus.
The ionization energy is likely to reduce as one proceeds downwards the periodic table because the electrons in the outermost shell are further away from the nucleus and more insulated. They are less attracted to the positive charge of the nucleus. Ionization energy increases from Hydrogen to Helium in a period but decreases from Hydrogen to Francium in a group.
CONCLUSION:
- The energy required to remove an electron from a certain atom is known as ionization energy
- The ease with which electrons can be withdrawn from an atom affects the atom’s reactivity.
- The ionization energy of electrons in different atomic or molecular orbitals is variable.
- Across the periodic table, ionization energy increases from left to right, and as we progress downwards in a group, the ionization energy decreases.
FAQs:
1. How is the first ionization energy trend?
The first ionization energy increases as you move from left to right over time, but it decreases as you move down a group.
2. What is the trend for ionization energy?
The general trend of ionization energy in a periodic table is that the energy increases as you move in the direction of hydrogen to helium and decreases as you move from hydrogen to cesium.
3. What is the trend in 1st ionization energy from top to bottom down a group?
The first ionization energy decreases as you go to the bottom of a group.
4. What is the group trend in the first ionization energies?
In general, ionization energy rises over a period and falls as a group descends.
*We hope you enjoyed studying this lesson and learned something cool about the Trends in the first ionization energy! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎 *
SOURCES:
- Periodic Trends: Ionization Energy. https://www.ck12.org/c/chemistry/periodic-trends:-ionization-energy/lesson/Periodic-Trends%3A-Ionization-Energy-CHEM/. Accessed 31 Jan 2022.
- Ionization Energy. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Ionization_Energy. Accessed 31 Jan 2022.
- Ionization Energy. https://courses.lumenlearning.com/introchem/chapter/ionization-energy/. Accessed 31 Jan 2022.