Balancing Redox Reactions: Half-Reaction Method in Basic Solution Study Guide
INTRODUCTION
Whenever we see a physical or chemical change in any object near us, we automatically conclude that some reaction has occurred. Have you ever wondered which reaction is taking place when we burn a matchstick?
In real life, combustion is a famous example of redox processes. When we think of combustion, though, we normally think of it as a physical rather than a chemical process. Another significant example of redox reactions is burning organic matter and hydrocarbons in fossil resources.
The oxygen in the atmosphere reacts with the carbon and hydrogen in the chemical being burnt, forming a bond. Oxygen in the atmosphere is reduced throughout combustion, while the chemical being burned is oxidized. Well now, we have talked a lot about redox reactions! But what exactly is a redox reaction?
WHAT IS A REDOX REACTION?
All chemical processes in which atoms’ oxidation states are altered are referred to as redox (oxidation-reduction) reactions. The loss of electrons—or the rise in oxidation state—by a molecule, atom, or ion is called oxidation. A molecule, atom, or ion gains electrons—or decreases its oxidation state—during reduction.
Every chemical reaction is represented in the form of equations and it is very important for both scientific and academic purposes that these equations are balanced.
BALANCING OF REDOX REACTIONS:
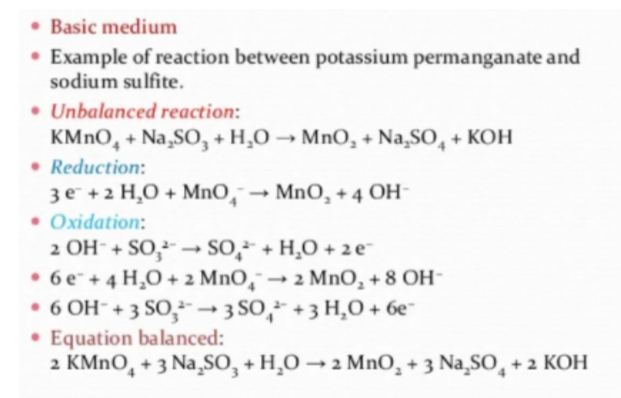
To balance the overall reaction in an alkaline medium, OH ions and water are introduced to half of the reactions. Take the interaction involving potassium permanganate and sodium sulfite, for example:
MnO₄– + SO₃²⁻ + H₂O → MnO₂ + SO₄²⁻ + OH–
The imbalanced reaction may be split into two halves, each of which represents reduction or oxidation.
- Reduction: 3 e− + 2 H₂O + MnO₄– → MnO₂ + 4 OH−
- Oxidation: 2 OH− + SO₃²⁻ → SO₄²⁻ + H₂O + 2 e−
STEPS TO BALANCING A REDOX REACTION:
- Write the imbalanced ionic equation.
- Separate half-reactions should be written for the oxidation and reduction events.
- Apart from hydrogen and oxygen, counterbalance the atoms in the half-reactions.
- Water molecules are added to the appropriate end of the equation to equalize oxygen atoms.
- The hydrogen atoms must then be balanced.
- By giving electrons to every other half-reaction, the charges are balanced.
- Then, by multiplying it all in one or even both equations by a coefficient, equalize the electrons.
- Combine the two half-reactions. The electrons must balance each other out. By inspecting the remaining ingredients, you may find out whether there are any that need to be balanced H2O or H that occur on both sides should be canceled if required.
- The hydroxide ions will then be added.
- Making water by merging hydrogen and hydroxide ions.
- To reach the final equation, eliminate the water molecules over both sides.
By following the above rules, we reach the final balanced reaction of potassium permanganate and sodium sulfite:
2 MnO₄– + 3 SO₃²⁻ + H₂O → 2 MnO₂ + 3 SO₄²⁻ + 2 OH–
CONCLUSION:
- All chemical processes in which atoms’ oxidation states are altered are redox reactions.
- The type of reaction determines which balancing approach should be used.
- The half-reaction approach for balancing redox is more adaptable, and it works well for ions in aqueous solution interactions.
FAQs:
1. How do you know if a redox reaction is acidic or basic?
It’s an acidic medium if H is present throughout the reaction’s balance, and it’s a basic media if OH- is present.
2. How do you balance redox reactions in acidic and basic mediums?
- Identify the half-reactions.
- Balance the components that aren’t O and H.
- Use H2O to restore oxygen equilibrium.
- Insert protons (H) to balance hydrogen.
- Use electrons to equalize the charge of each equation.
- Equalize the electrons in the processes by scaling them.
3. What is a redox reaction?
Redox reactions are oxidation-reduction chemical processes in which the oxidation states of the reactants change.
We hope you enjoyed studying this lesson and learned something cool about Balancing redox reactions! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Balancing Redox Reactions: Half-Reaction Method in Basic Solution. https://www.ck12.org/c/chemistry/balancing-redox-reactions:-half-reaction-method-in-basic-solution/lesson/Half-Reaction-Method-in-Basic-Solution-CHEM/. Accessed 27 Jan, 2022.
- Balancing Redox Equations. https://courses.lumenlearning.com/introchem/chapter/balancing-redox-equations-2/. Accessed 27 Jan, 2022.

