Corrosion Study Guide
INTRODUCTION
Corrosion is a prevalent occurrence in metal items and surfaces. It occurs when metal reacts with substances in the environment, causing slow disintegration. It erodes the metal, leaving visible holes and altering the chemical composition. While all types of corrosion have this impact, galvanic corrosion is especially troublesome because it can induce different levels of damage between two metals.
WHAT IS GALVANIC CORROSION?
- Galvanic corrosion, also called bimetallic erosion, occurs when two different metals are exposed to an electrolyte.
- When this happens, one of the metals starts to rust, whereas the other remains safe.
- The node is the metal that erodes throughout galvanic corrosion. The cathode is a metal that does not rust.
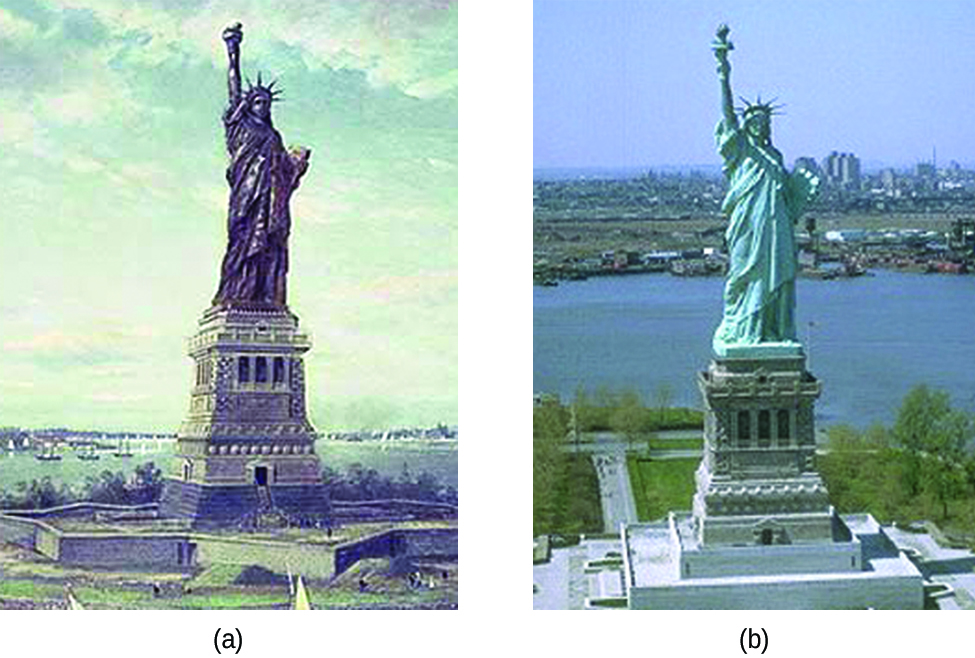
- The Statue of Liberty is a well-known case of galvanic corrosion.
STATUE OF LIBERTY CORROSION
- The statue of liberty wasn’t green when it was first shipped from France. The color of its copper “skin” was brown. So, how did the hue of the Statue of Liberty change? Corrosion was the cause of the color change.
- The copper, which is the statue’s main component, oxidized over time due to exposure to the air.
- The copper metal undergoes a series of oxidation-reduction reactions in the environment.
- Copper metal oxidizes to copper(I) oxide, a reddish-brown color, and subsequently to copper (II) oxide, a blackish-brow color.
2Cu(s)+ ½ O₂(g) ⟶ Cu₂O(s) (red)Cu₂O(s)+ ½ O₂(g) ⟶ 2CuO(s) (black)
- Coal, frequently heavy in sulfur, was widely burned in the early twentieth century. Sulfur trioxide, carbon dioxide, and water all interacted with the CuO as a consequence.
2CuO(s) + CO₂(g) + H₂O(l) ⟶ Cu₂CO₃(OH)₂(s) (green)3CuO(s) + 2CO₂(g)+ H₂O(l) ⟶ Cu₂(CO₃)₂ (OH)₂(s) (blue)4CuO(s) + SO₃(g) + 3H₂O(l) ⟶ Cu₄SO₄(OH)₆(s) (green)
- The striking blue-green patina visible today is due to these three chemicals. Fortunately, the patina formed a protective covering on the exterior, preventing the copper skin from eroding further.
CONCLUSION
- The Statue of Liberty is a prominent example of galvanic corrosion.
- In conjunction with the copper skin, the Statue of Liberty’s iron framework created a classic galvanic corrosion condition.
- The exterior of the Statue of Liberty is composed of copper, which has oxidized to a shade of green.
FAQs:
1. Is the Statue of Liberty corroded?
The node is the metal that corrodes throughout galvanic corrosion. The Statue of Liberty is a typical example of galvanic corrosion.
2. What causes the corrosion of the Statue of Liberty?
In conjunction with the copper skin, the Statue of Liberty’s iron framework created a classic galvanic corrosion condition. When two or more metals are electrically linked in the same electrolyte, this form of deterioration happens.
3. Will the Statue of Liberty continue to erode?
Despite all efforts to protect the monument, copper and iron cannot resist the “radical” character of oxygen, and the Statue of Liberty will never be free of corrosion.
4. Why is the Statue of Liberty green?
The outside of the Statue of Liberty is composed of copper, which has oxidized to a shade of green.
We hope you enjoyed studying this lesson and learned something cool about the Corrosion! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Preserving the Statue: https://www.ck12.org/c/chemistry/corrosion/rwa/Preserving-the-Statue/ Accessed 27 Feb 2022
- Corrosion: https://courses.lumenlearning.com/chemistryatomsfirst/chapter/corrosion/ Accessed 27 Feb 2022.