Identifying Redox Reactions Study Guide
INTRODUCTION
A new object made of iron is initially grey, but it will develop a reddish appearance if left in the open for a long time. This is due to the oxidation of iron, also known as rusting. This is a typical example of the redox reaction that we see in our everyday lives. So, are you wondering what a redox reaction is? 🤔
REDOX REACTIONS:
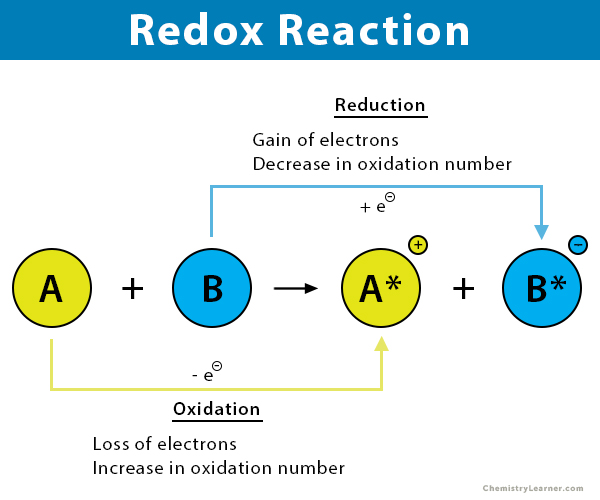
Redox reactions are oxidation-reduction chemical processes in which the oxidation states of the reactant changes. (The degree of oxidation for an atom in a chemical compound is indicated by its oxidation state, which is the imaginary charge that an atom would have if all links to atoms of various elements were entirely ionic.)
- The loss of electrons or a rise in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as oxidation.
- The gaining of electrons or a drop in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as reduction (a reduction in the oxidation state).
- In a redox reaction, the oxidation and reduction processes always happen simultaneously.
IDENTIFYING REDOX REACTION:
Because two separate elements appear as free elements (oxidation number of 0) (zinc and copper in the example below) on one side of the equation and as components of a molecule on the other, single-replacement processes like the one given below, are redox reactions. As a result, the oxidation number of the substance must change.
Zn (s) + CuSO₄ (aq) → ZnSO₄ (aq) + Cu (s)Oxidation half-reaction: Zn → Zn²⁺ + 2e–
Reduction half-reaction: Cu²⁺ + 2e– → Cu
Because elemental oxygen (O2) functions as the oxidizing agent and is reduced, combustion processes are redox reactions.
S(s) + O₂ (g) → SO₂ (g)Because elements are generally changed into compounds, and vice versa, most combination and decomposition processes are redox reactions.
2H₂O → 2H₂ + O₂Here, H+ is oxidized, and O- is reduced.
NOW LET’S LOOK AT A FEW REACTIONS THAT ARE NOT REDOX:
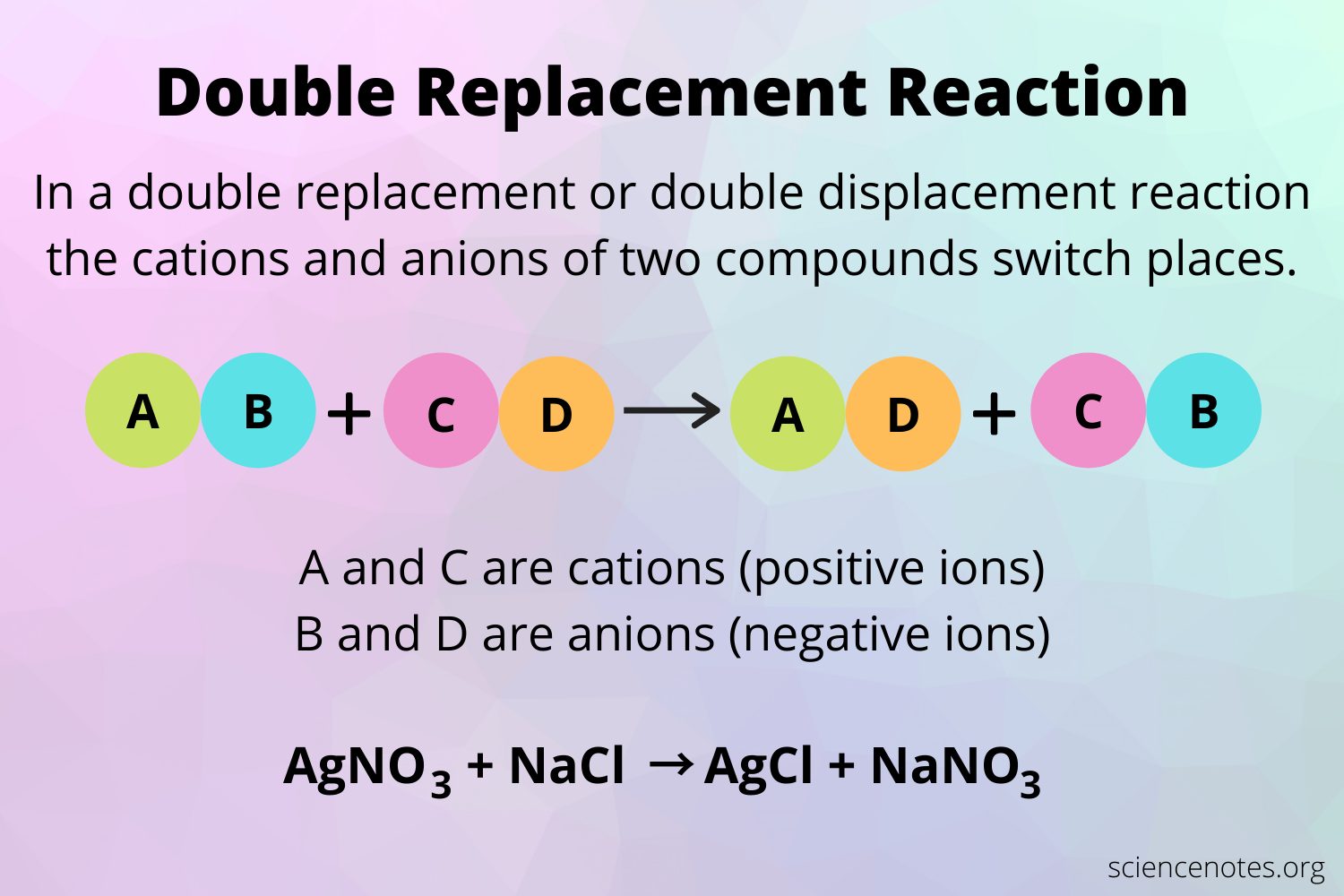
Because ions are merely merged without any electron transfer, double-replacement events like those below aren’t redox reactions.
BaCl₂ (aq) + Na₂SO₄ (aq) → BaSO₄ (s)+ 2NaCl(aq)If you calculate the oxidation numbers on all the ions, you will realize that the oxidation number of all the elements remains unchanged after the reaction.
Instead of an electron, a hydrogen ion is transferred in acid-base reactions. Acid base reactions, such as the one beneath, are not redox reactions.
CH₃COOH + NH₃ ⇌ CH₃COO– + NH₄+CONCLUSION
- Redox reactions are oxidation-reduction chemical processes in which the oxidation states of the reactant changes.
- Decomposition, combustion, Disproportionation, Displacement & Combination Reactions are the 5 major types of redox reactions.
- Because ions are merely recombined without any electron transfer, double-replacement processes are not redox.
FAQs:
1. What is the definition of redox?
All chemical processes in which atoms’ oxidation states are altered are redox (oxidation-reduction) reactions. The loss of electrons—or the rise in oxidation state—by a molecule, atom, or ion is called oxidation. During reduction, a molecule, atom, or ion gains electron—or decreases its oxidation state.
2. What is an example of redox reaction?
H₂ + F₂ → 2HF. Following that, the hydrogen and fluorine ions unite to produce hydrogen fluoride. F₂ is an oxidizing agent, whereas H₂ is a reducing agent in this reaction.
3. What types of reactions are redox reactions?
Decomposition Reaction, combustion reactions, Disproportionation Reaction, Displacement Reaction & Combination Reactions are the 5 major types of redox reactions.
We hope you enjoyed studying this lesson and learned something cool about Identifying Redox Reactions! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Identifying Redox Reactions. https://www.ck12.org/c/chemistry/identifying-redox-reactions/lesson/Identifying-Reaction-Types-CHEM/. Accessed 7 Feb 2022.
- Acid-Base Reactions. https://courses.lumenlearning.com/boundless-chemistry/chapter/acid-base-reactions/. Accessed 7 Feb 2022.