Colligative Properties Study Guide
INTRODUCTION
Those in colder areas have seen the trucks spread salt on the roadways. Why? When flying in frigid weather, planes must be de-iced before take-off. Why is that the case? Pure solvents have different boiling and freezing points than solutions. All the colligative properties (like vapor pressure, boiling, and freezing temperature) and their values change by adding a solute to an existing solution.

WHAT ARE COLLIGATIVE PROPERTIES?
- Colligative qualities are those of a solution determined by the number of particles in a given volume of solvent (concentration) rather than the mass or nature of the solute particles.
- The temperature has an impact on colligative characteristics as well.
- Vapour pressure reduction, freezing point depression, osmotic pressure reduction, and boiling point elevation are colligative qualities.
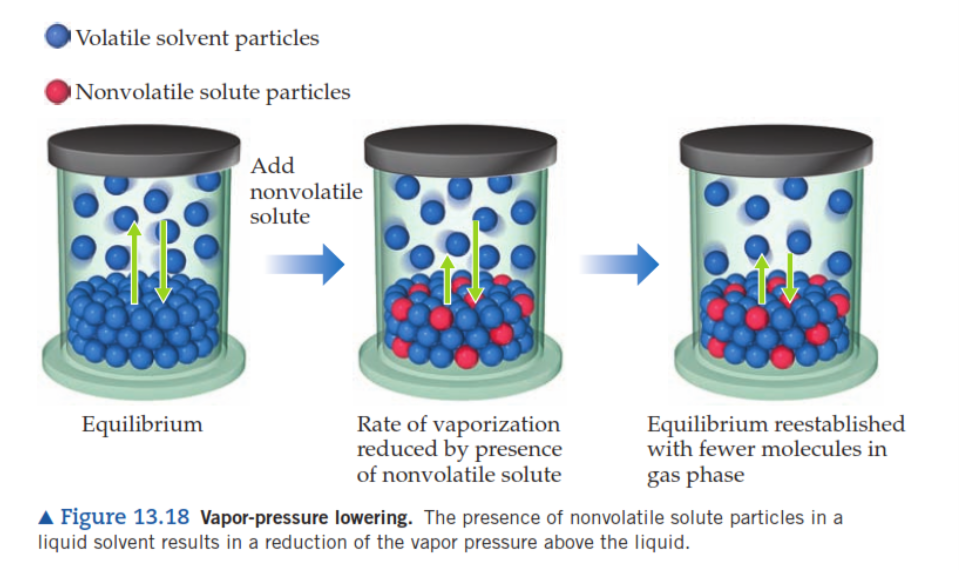
VAPOR PRESSURE LOWERING
- The pressure imposed by a liquid’s gaseous form when vaporization and condensation happen at equal rates is known as the equilibrium vapor pressure:Liquid ⇌ gas
- When a non-volatile chemical is dissolved in a volatile liquid, the vapor pressure of the liquid is reduced.
- Solvent molecules have to be located at the solution’s surface for it to evaporate.
- The presence of a solute limits the accessible surface area for solvent molecules, lowering the rate of solvent vaporization.
- Because the availability of a solute does not affect the rate of condensation, the vaporization-condensation equilibrium is attained with fewer solvent molecules in the vapor phase (i.e., at a lower vapor pressure).
Colligative Properties Example
The examples below show the colligative properties of Osmotic pressure and Vant Hoff factor.
Osmotic Pressure
The spontaneous uni-directional flow of solvent from lower concentration to higher concentration solution through semipermeable membrane is called as osmosis. The excess of pressure on the side of solution that stops the net flow of solvent from lower concentration to higher concentration solution via semi permeable membrane is called as osmotic pressure.
= CRT (n/V)RTV = nRT
where C is concentration, R is gas constant, and T is temperature.
Since osmotic pressure depends on the number of moles, it is a colligative property.
Vant Hoff Factor (i)
It is defined as the ratio of the observed colligative property produced by a given concentration of electrolyte solution to the property observed for the same concentration of non-electrolyte solution.
i = observed colligative property of the electrolyte/observed colligative property of the non-electrolyte
The observed colligative property of non-electrolyte is called the theoretical colligative property.
Therefore i = observed colligative property/theoretical colligative property
Since we know that the colligative property is directly proportional to the number of solute particles.
Therefore, i = n (observed value)/n (theoretical value). Here n is the number of moles or the number of particles. The theoretical value would be a fixed value, while the observed value would be more or less depending on the association or dissociation. And Colligative property is inversely proportional to molar mass.
Therefore, i = M (theoretical)/M (observed). Here, M stands for the molar mass of the substance.
BOILING POINT ELEVATION
The temperature at which a liquid’s vapor pressure equals ambient air pressure is its boiling point. Because the introduction of non-volatile solutes lowers a solution’s vapor pressure, it logically follows that the solution’s boiling point will rise as a result.
The elevation of the boiling point can be estimated using the equation: ΔTb = Kb*m
where,
Kb = ebullioscopy constant
m = molality of the solute
ΔT = Change in temperature
FREEZING POINT DEPRESSION
-
Solutions freeze at lower temperatures as compared to pure liquids. This phenomenon is used in “de-icing” schemes that employ salt, calcium chloride, or urea to melt the ice on streets and sidewalks and in the use of ethylene glycol as an “antifreeze” in car radiators.
-
The lowering in freezing point can be estimated by using:
ΔT = i*Kf*m
where,
ΔT = Change in temperature
i = van ‘t Hoff factor
Kf = molal freezing point depression constant or cryoscopy constant
m = molality of the solute
CONCLUSION
- Colligative qualities are those of a solution solely dependent on the concentration of solute molecules.
- The four colligative properties are vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
FAQs:
1. What are the four colligative properties?
The four colligative properties are vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
2. Which are the Colligative properties?
Colligative characteristics are those of a solution determined only by the number of particles of the solute, such as ions or molecules, in a given quantity of the solvent rather than the type of the solute.
3. What are colligative properties? Give examples.
Compound attributes that are affected by the concentration of the substance present are known as colligative properties. The four primary colligative qualities are the boiling point, freezing point, vapor pressure, and osmotic pressureare the four primary colligative qualities.
We hope you enjoyed studying this lesson and learned something cool about Colligative Properties! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>