Osmotic Pressure π = MRT Study Guide
INTRODUCTION
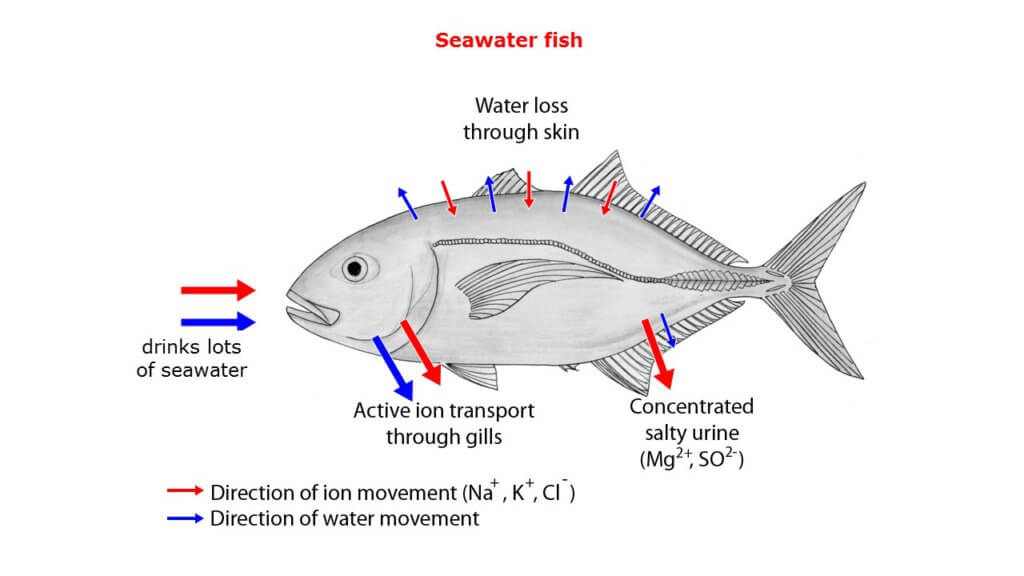
The membranes of fish cells, like those of other cells, are semi-permeable. The concentration of “things” along either side of them should eventually balance out. Inside a fish that thrives in salt water, there will be some salty water. If you put it in freshwater, the freshwater will enter the fish through osmosis, prompting its cells to enlarge and the fish to die. If we want to stop the water from moving along its natural tendency, we’ll have to apply some external pressure to stop it. This pressure is referred to as osmotic pressure. Let’s take a closer look at this.
WHAT IS OSMOSIS?
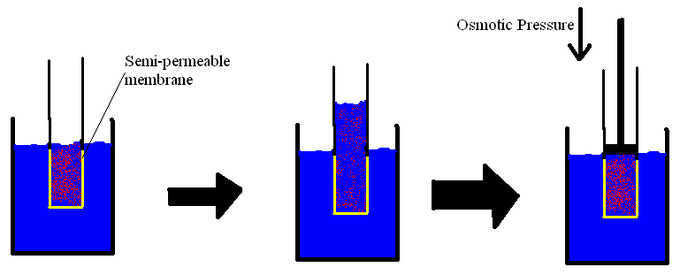
The overall movement or flow of solvent molecules over a semi-permeable membrane through which solute particles cannot pass is called osmosis.
OSMOTIC PRESSURE
- The pressure that must be provided to a solution to avoid the inward movement of water through a semipermeable membrane is known as osmotic pressure.
- The pressure required to nullify osmosis is also known as osmotic pressure.
- Increasing the hydrostatic pressure on the solution side of the membrane, which squeezes the solvent molecules nearer and increases their “escaping tendency,” is one technique to limit osmosis.
- The solution’s escape tendency can be increased until it reaches the same level as the particles in the pure solvent; at this juncture, osmosis will stop.
FORMULA TO CALCULATE OSMOTIC PRESSURE
The osmotic pressure is the amount of pressure needed to achieve osmotic balance.It is a colligative feature influenced by the solute molecule concentration in the solution. The following equation can be used to compute osmotic pressure:
- π = iCRT
- π – Osmotic pressure
- i – Van’t Hoff factor
- C – Molar concentration of the solute in the solution
- R – Universal gas constant
- T – Temperature
REVERSE OSMOSIS:
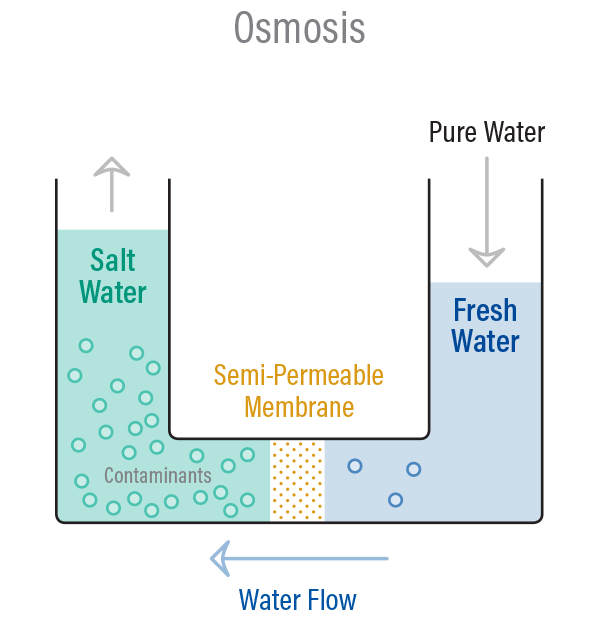
Now you may wonder what happens when the solution side is subjected to a pressure greater than the osmotic pressure?In this case, the solvent molecules would begin to move from the solution side (where the solute levels are high) to the solvent side thru the semi-permeable barrier (where the solute concentration is low). This is known as reverse osmosis.
CONCLUSION:
- The overall movement or flow of solvent molecules over a semi-permeable membrane is called osmosis.
- The pressure that must be provided to a solution to avoid the inward movement of water through a semi-permeable membrane is known as osmotic pressure.
FAQs:
1. What is pi in osmotic pressure?
Π is the osmotic pressure in atm. Water moves towards the solution with the greatest concentration due to osmotic pressure.
2. What is MRT in chemistry?
Pi=MRT, wherein Pi is the osmotic pressure in atm, M is the molarity, R is the ideal gas constant, and T is the kelvin temperature, is used to determine the osmotic pressure of a solution.
3. What is pi = CRT?
The equation π = CRT gives the osmotic pressure of a solution (where C is the molarity of the solution).
We hope you enjoyed studying this lesson and learned something cool about Osmotic Pressure π = MRT! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Osmotic Pressure: https://courses.lumenlearning.com/introchem/chapter/osmotic-pressure/ Accessed 26 Feb 2022
- Osmotic Pressure Equation: https://byjus.com/chemistry/osmotic-pressure-equation/ Accessed 26 Feb 2022