Rate of Dissolving: Dissolving Sugar Study Guide
Introduction
Have you ever wondered what happens to sugar when we mix sugar into the water? Why does it disappear? But as we keep on mixing sugar to the same solution, it ceases to disappear? What if we heat it further?Let’s find answers to all the questions!
SOLUBILITY
Solubility is defined as the maximum amount of substance that can be dissolved in a solvent at some particular temperature.
- In this picture, as you can see, after mixing sugar, it disappeared as it has been dissolved.
- This means that sugar is soluble in water.
- Do you know that 1800 grams of sugar can be dissolved in 1 L of water at 25℃? This means these numbers define the solubility of sugar in water.
- But what happens when we keep on dissolving sugar into the water?
- No more sugar can be dissolved after a certain point as now it becomes a saturated solution.
WHAT IS A SATURATED SOLUTION?
- A solution in which no more solute can be dissolved any further without increasing the temperature of the solution is a saturated solution.
- When we keep on adding sugar to water, there comes the point where no more sugar can be dissolved as it starts setting at the bottom of the container.
- This happens because the rate of dissolution becomes equal to the rate of crystallization.
But can we dissolve the sugar that has been settled at the bottom of the container?Yes! But how?
FACTOR AFFECTING THE SOLUBILITY
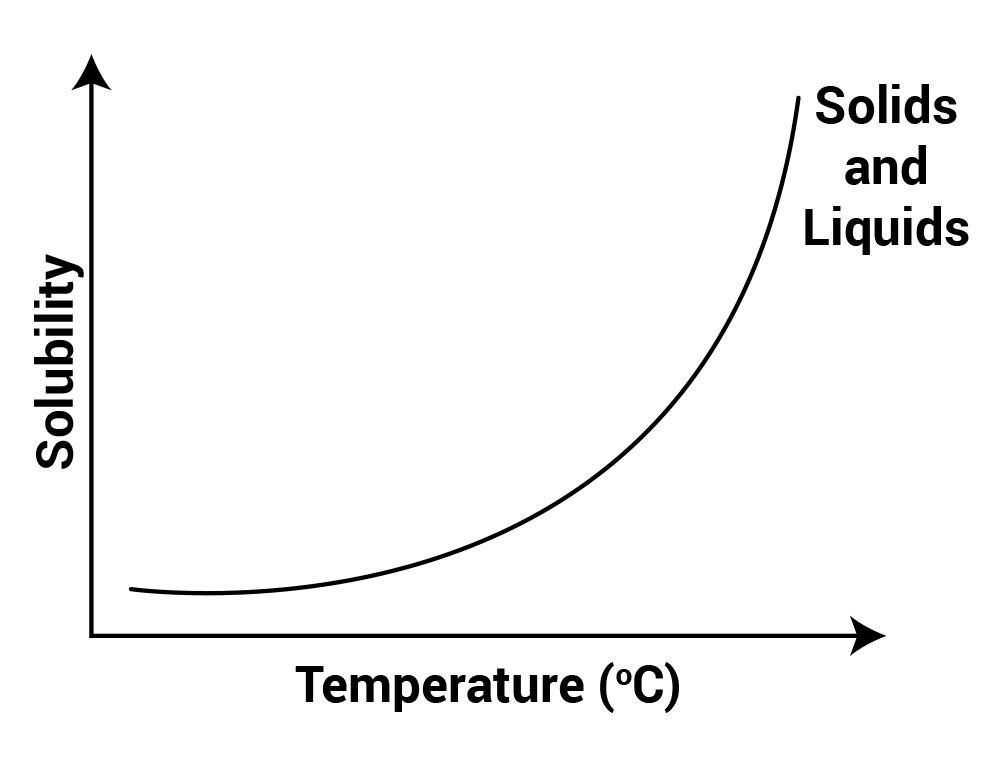
Temperature affects the solubility of a substance in a solvent.
On increasing temperature, the solubility of solid substances increases because energy is required to break the bonds in solid, and when that energy is supplied in the form of heat, bonds break and solubility increases.
So, when no more sugar can be dissolved in a container, that is when the saturated solution is formed; just heat the solution to dissolve the sugar.
But can we do the same for gases? Does the solubility of gases increase by increasing the temperature of the solution?No!
SOLUBILITY OF GASES
- Increasing the temperature increases the kinetic energy of gases.
- The gas molecules will try to escape the liquid as intermolecular forces between gas molecules will break in which they are dissolved, which means solubility will decrease.
- This is exactly the opposite of the dissolution of solids in liquid.
CONCLUSION
- Solubility is defined as the maximum amount of substance that can be dissolved in a solvent at some particular temperature.
- Solubility increases by increasing the temperature in the case of solids, but the trend is the opposite for gases.
FAQs
1. What is solubility?
Solubility is defined as the maximum amount of substance that can be dissolved in a solvent at some particular temperature.
2. What is a saturated solution?
A solution in which no more solute can be dissolved any further without increasing the temperature of the solution is a saturated solution.
3. Does increasing the temperature increase the solubility of a solid substance?
Yes, as on the increasing temperature, the solubility of solid substances increases because energy is required to break the bonds in solid and thus solubility increases.
We hope you enjoyed studying this lesson and learned something cool about the Rate of Dissolving! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
SOURCES
- Rate of Dissolving: https://www.ck12.org/assessment/tools/geometry-tool/plix.html?eId=SCI.CHE.754&questionId=53d7e8538e0e087ba8b8e938&artifactID=1886265&conceptCollectionHandle=chemistry-::-rate-of-dissolving&collectionCreatorID=3&plix_redirect=1.Accessed 10th March 2022.
- How Much Sugar Can Water Dissolve: https://lisbdn`et.com/how-much-sugar-will-dissolve-in-1000-g-of-water-at-90ac/#:~:text=ground%20water%20level-,How%20much%20sugar%20can%20water%20dissolve%3F,is%20said%20to%20be%20saturated.Accessed 10th March 2022.
- General Solubility of Gases: https://www.toppr.com/ask/question/generally-solubility-of-gases-in-liquids-is-decreases-as-increasing-temperature-give-reason/.Accessed 10th March 2022