Solubility Product Constant Study Guide
INTRODUCTION
Have you ever wondered how the solid cubes of sugar dissolve so quickly in water? The solid cubes break down into individual molecules and are separated by the water molecules surrounding them. The solubility of a substance can be defined as the property of solute to dissolve in a solvent to form a solution. Every solvent has a solubility product, notated as Kₛₚ.
WHAT IS Kₛₚ IN CHEMISTRY?
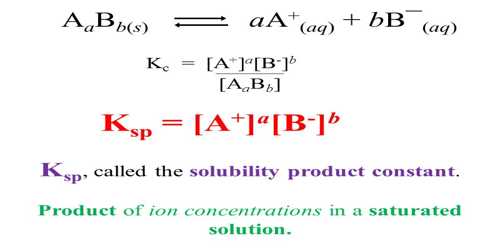
The solubility product constant, or Ksp, is an equilibrium constant of a solute that can dissolve easily in a solvent, for example, dissolving a sugar cube in water. In this scenario, the glucose that makes up the sugar is the solute, and the water is the solvent. As we mix them together, the glucose particles completely dissolve in water. Ksp is the constant number that describes the molar changes that occurred with the dissolution of glucose in water. Let’s take a look at an example of an aqueous solution:
bB (s) ⇆ eE (aq) aA (aq)
Here,
B = Solid
E, A = aqueous medium after the solid dissolves in it
We can find out the value of Ksp by multiplying the product if the molarities of the products. If there are coefficients present in the product, then we need to raise the product to the power of the coefficient and then multiply them to get the Kₛₚ. So,
Kₛₚ = [E]e x [A]a
Here, you will not find bB, as solid is not calculated in Kₛₚ. Hence, the solubility product constant can only be calculated in the aqueous medium.
PURIFYING WATER WITH THE HELP OF SEASHELLS
Natural sources of water have a high concentration of metal cations. These metals cations are toxic to humans and thus make the water unsuitable for drinking. To overcome this problem, many countries use advanced technology to filter these heavy metals from the water. Others without access to such technology use a different but still effective method: seashells.
66.70% of a seashell is composed of aragonite, a form of calcium carbonate (CACO3). Seashells help clean water by swapping calcium atoms with heavy metals, effectively filtering the water by locking them into a solid form.

Significance of Solubility Product
• When a salt dissolves in a solvent, the interactions between the ions and the solvent must overcome the strong forces of attraction of the solute, which are the lattice enthalpies of its ions.• As ions always have a negative solvation enthalpy, energy is released throughout the process.• The solvation enthalpy, or the amount of energy released during solvation, is determined by the composition of the solvent.• The solvation enthalpy of non-polar solvents is low, which indicates that it is insufficient to overcome the lattice enthalpy.• Because of this, the salts cannot be dissolved in non-polar solvents. As a result, a salt’s solvation enthalpy must be higher than its lattice enthalpy in order for it to dissolve in a solvent.• Every salt has a different solubility that varies with temperature.
Solubility Product Constant
The ability of a material, known as a solute, to dissolve in a solvent to create a solution is known as solubility. Ionic compounds that separate into cations and anions in water have a wide range of solubilities. Other compounds, on the other hand, are very insoluble. Some chemicals are highly soluble and may even absorb moisture from the air.
Temperature-dependent equilibrium constants include the solubility product. Because of the higher solubility, Ksp often rises as temperature rises. Even the majority of insoluble ionic compounds would partially dissolve in water. Since the dissolved fraction of these insoluble compounds also dissociate, they are regarded as strong electrolytes. For instance, when introduced to water, silver chloride dissociates a minor amount into silver ions and chloride ions.
Solubility Product Formula
The saturated solutions of ionic substances with relatively poor solubility are described by the solubility product constant. The ionic component and the undissolved solid are said to be in a condition of dynamic equilibrium in a saturated solution.
The following equation serves as the representation of the Ksp formula:
MxAy (s)→xMy+ (aq)+yAx- (aq)
The formula for the global equilibrium constant is as follows:
Kc=[My+]x [Ax-]y
FAQs
1. How do you find the solubility product constant Kₛₚ?
The solubility product constant Ksp is the equilibrium constant for a solid substance that dissolves in a liquid or aqueous medium. The more soluble the solid is, the higher will be its Kₛₚ value.
2. How is solubility related to Kₛₚ?
Ksp is the product of each ion in moles per liter. The relation of solubility and the solubility product constant is used to find the value of each other through the formation of the equation.
3. What is Kₛₚ equal to?
Kₛₚ = product of ions
4. What is the relationship between the solubility product constant Kₛₚ and temperature?
The solubility product Ksp increases with the increase in temperature. The increase in temperature will help the solute to break down and dissolve in the solvent faster. Hence, the Ksp increases with the temperature increase.
We hope you enjoyed studying this lesson and learned something cool about Solubility Product Constant! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>