Supersaturated Solution Study Guide
INTRODUCTION
We all know how refreshing a cold glass of lemonade is in the exhausting summer season. The lemonade we make is an ideal example of a solution in chemical terms. A solute, which is relatively lower in quantity, is added into a greater quantity of a solvent in a solution. In the case of lemonade, the water is the solvent (as it is present in a greater quantity), and the sugar and the lime juice are the solutes that get dissolved in the solvent. But what happens if we continue to add the solutes in our solvent? 🤔
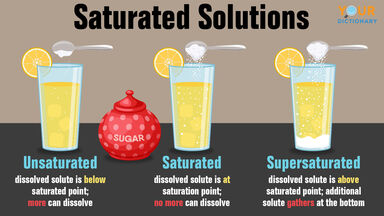
What is the Solution?
In chemistry, a solution is a homogeneous combination of two or more compounds in their relative proportions. The composition of the solution can be continually changed up to the solubility limit. Although solutions of gases and solids are possible, the term “solution” is most frequently used to refer to the liquid condition of matter. Brass is a solution made of copper and zinc, whereas air is mostly an oxygen and nitrogen mixture with minute quantities of other gases.
WHAT IS A SUPERSATURATED SOLUTION IN CHEMISTRY?
A supersaturated solution has gone above saturation. When the solute in a liquid is in a stable system with the non-soluble form of the solute, the solution is said to be saturated. When a solution’s solute concentration reaches its maximum potential concentration, it is saturated. On the other hand, a supersaturated solution has more absorbed solute than a saturated solution.
A supersaturated solution of Sodium Acetate would be an ideal example. We know that sodium acetate does not recrystallize easily. No crystals appear when a saturated sodium acetate solution is cooled down, but surplus dissolved solute crystallizes when a small quantity of solute crystals are seeded into a supersaturated solution. This recrystallization process is achieved because of the supersaturated sodium of acetate solution.
Supersaturation in Phase Change
• Every system’s vapour melt or solution phase is where physical and chemical processes take place. These processes only happen when the medium is supersaturated, and they involve the production of three-dimensional (3D) nuclei of a new phase.
• A change in the system’s free energy is connected to the nuclei’s creation. Even if thermodynamically such a condition is feasible, in the homogeneous system nuclei of the new phase do not develop as soon as the system becomes supersaturated.
• Without obtaining the least free energy corresponding to the equilibrium state, the system is said to be in a condition of metastable equilibrium.
• In other words, in these situations, the process of nucleation of the new phase is facilitated by increased supersaturation level, chemical phases other than the nucleating phase, and temperature and pressure of the system. The value relies on these parameters.
• The next phase nucleates instantly, although there is always a supersaturation level. That is where the next phase begins.
• This supersaturation level establishes the metastable width and corresponds to the upper bound of the metastable equilibrium condition.
CHARACTERISTICS OF A SUPERSATURATED SOLUTION
- Concentration of solute surpasses its solubility
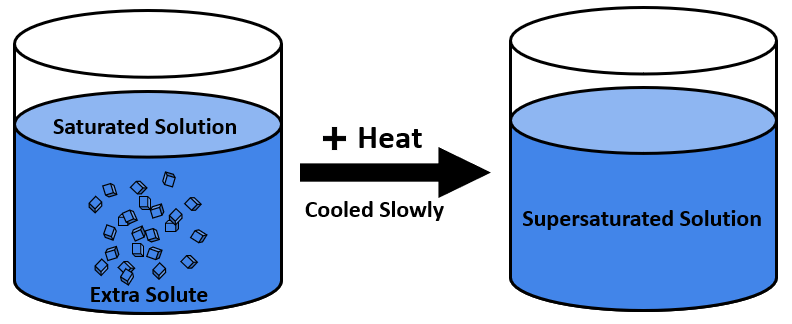
A solute’s solubility tends to increase as the temperature is raised. As a result, if a saturated solution produced at a high temperature is refrigerated, it will become a supersaturated solution as long as crystallization and condensation processes are prevented.
- Metastable solution
In a supersaturated solution, the extra solute represents the extra energy. In other words, a supersaturated solution is stuck in an energy state more than its normal condition because it is not in dynamic equilibrium.
- Crystallization
Since the crystallization and condensation activities ordinarily bring the solutions into dynamic equilibrium are kinetically prevented, supersaturated solutions arise. The heat required to form these crystals and droplets is unavailable in some biochemical conditions, such as cooling a supersaturated solution. In such circumstances, adding seed crystals is the only method to get around the kinetic restrictions.
Applications of Supersaturated Solution
A solution of a solid solute dissolved in a liquid solvent is in thermodynamic equilibrium when it reaches saturation. The system must move into a non-equilibrium state, where the concentration of the solute in the solution exceeds its equilibrium concentration under the specified solution conditions, for crystallization to take place. Supersaturation is a term used to describe solutions that are out of equilibrium.
Cooling is the easiest and most popular way to create supersaturated solutions, but there are other ways as well, including solvent evaporation, temperature changes, pH changes, chemical reactions, and changes in the solvent’s chemical makeup.
Supersaturated Solution Examples
Clear honey contains supersaturated sugars. At normal temperatures, crystals typically form slowly, but cooling honey will cause sugar to dissolve fast.
Supersaturation is necessary for crystals to form when a solute is dissolved in water. The material must first be dissolved in hot or warm water. The solution becomes supersaturated as it cools to room temperature. A seed crystal is added to encourage crystal development. Otherwise, tiny contaminants in the solution or surface flaws on the container serve as nucleation sites.
CONCLUSION
- A supersaturated solution has more absorbed solute than a saturated solution
- It can be made by boiling a saturated solution, putting additional solute, and slowly reducing its temperature.
- A solute’s solubility tends to increase as the temperature is raised.
- As a result, if a saturated solution produced at a high temperature is refrigerated, it will become a supersaturated solution as long as crystallization and condensation processes are prevented.
FAQs
1. What is a supersaturated solution? Give an example?
A supersaturated solution has more dispersed solute than the maximum quantity of solute that may be dispersed at a specific temperature. A heat pack is an ideal example of a supersaturated solution made up of material such as sodium acetate.
2. What are the characteristics of supersaturated solutions?
The major characteristics of a supersaturated solution are
(1) The concentration of the solute surpasses its solubility
(2) the solution is metastable (i.e., not in equilibrium)
(3) crystallization and condensation activities are kinetically suppressed
3. What are a supersaturated solution and an unsaturated solution?
A supersaturated solution has more dispersed solute than the maximum quantity of solute that may be dispersed at a specific temperature. In contrast, the amount of solute that can be dissolved in an unsaturated solution is less than the maximum amount.
4. How is a supersaturated solution formed?
Supersaturated solutions are generally formed by adding more solute in a heated solution.
We hope you enjoyed studying this lesson and learned something cool about Supersaturated Solution! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
]]>