Particle Diagram Study Guide
It’s not miraculous, yet still surprising to think that, in a way, the rock-hard ice, the soft water, or the unnoticeable air are all forms of water, yet they tend to be so different. The basic nature of all these different phases of water is the same, but the only difference is how the particles are packed up. Lego toys can be another wonderful example. Like a building block, a lego can be put in countless ways to make countless objects. It all depends upon their packing.
PARTICLE MODEL
Even with the most powerful microscope, we can’t see the particles that make up everything around us. These particles are organized and move differently in each of the three states of matter. For example, water particles seem to be the same size, shape, and chemical makeup, whether solid (ice) or gaseous (steam), but how they travel and are ordered differs depending on the condition.
The four main principles of the particle diagram are:
- Particles make up all substances.
- The particles are drawn to one another (some strongly, others weakly).
- The particles are in motion (have kinetic energy).
- The particles travel more as the temperature increases (their kinetic energy increases).
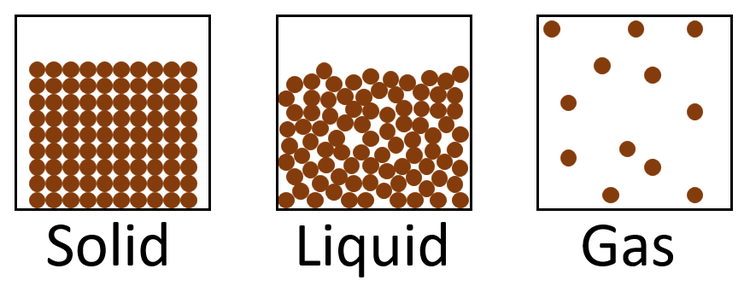
SOLIDS
The particles are grouped in a regular arrangement with very little space between them (think army ranks). Powerful forces keep their particles connected, and they can only oscillate around a fixed point. Solids have a high density and a definite volume and dimension.
The particle diagram of a solid has the following properties:
- They are closely packed.
- They are arranged.
- Particles do not overlap.
- Particles are so the same radius.
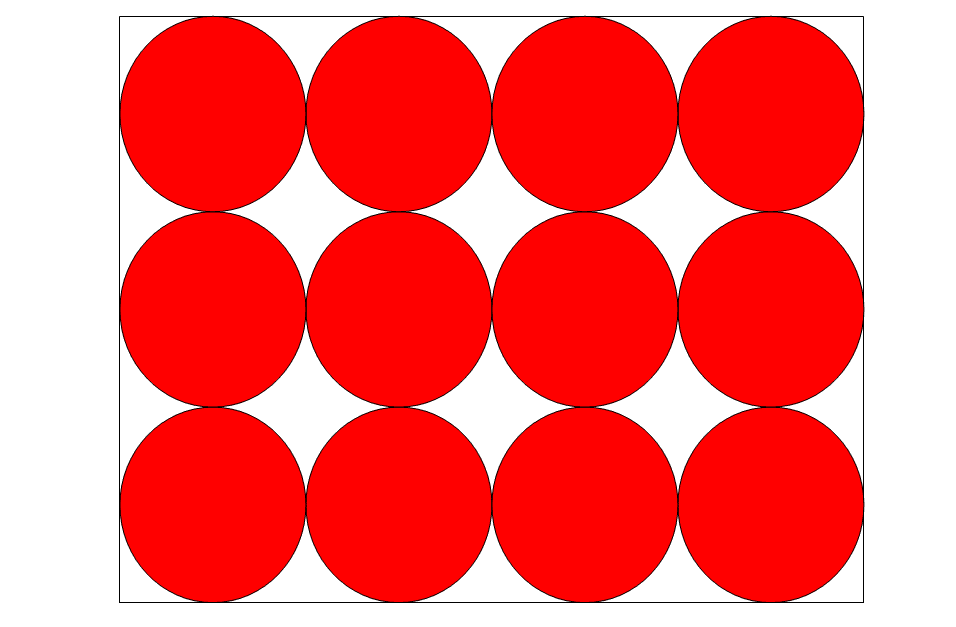
LIQUIDS
Liquids, just as solids, have a set capacity but not a definite shape. The liquids will flow to take on the form of the bottle’s bottom. Although all liquids can flow, some are slightly thicker than others. The stronger the tensions between the liquid molecules, the heavier (more viscous).
The particle diagram of the liquid has the following properties:
- Particles are present in a concentration at the bottom.
- All particles are in contact with one another.
- Slots between particles are too narrow to accommodate another particle.
- By pushing on other particles, particles construct ‘links’ over gaps.
- Particles are placed in such a way that they cannot be crushed into a smaller space.
- The particles are all the same size.
- There are no overlaps.
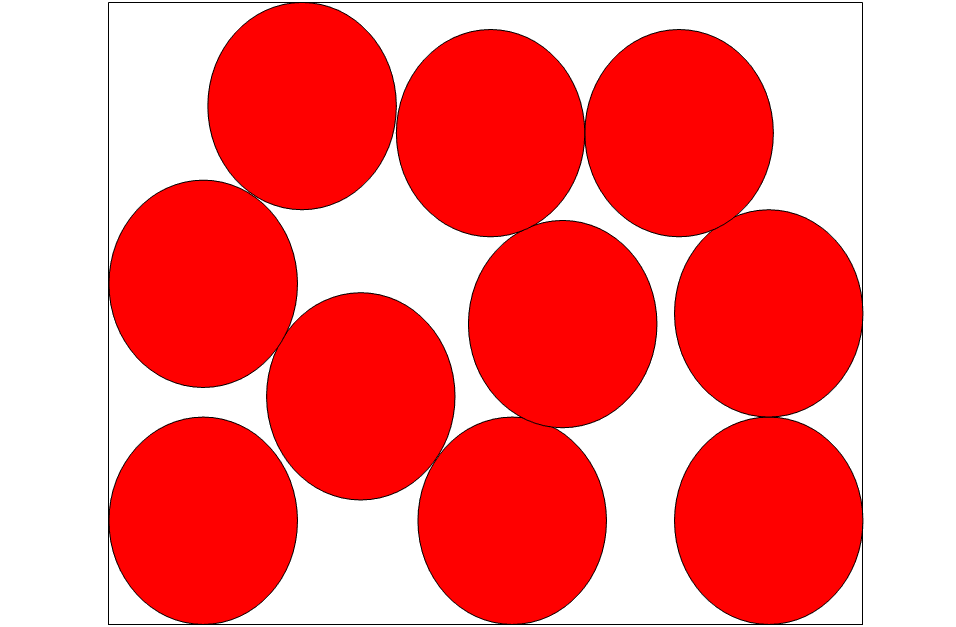
GASES
The particles in gases are spread out and organized in a random pattern. The kinetic energy of this form of matter is the greatest of the three traditional states, and there are essentially no bonds between the particles. The particles constantly travel on all sides, slamming into each other and the container’s sides, resulting in pressure.
The particle diagram of gases has the following properties:
- The particles are dispersed at random.
- The particles do not have a ‘dice pattern.’
- Particles can be seen moving in parallel lines (arrows).
- Particles are of the same diameter.
- There are no overlaps.
CONCLUSION
- The basic nature of all molecules in all three different phases is the same, but the only difference is how the particles are packed up.
- These particles are organized and move differently in each of the three states of matter.
- How the particles travel and are ordered differs depending on their current state.
FAQs:
1. What is a particle diagram?
The diagrammatic representation of elements and compounds and their composition with different types of balls and how they connect is called a particle diagram.
2. How do you draw a particle diagram?
A particle diagram can be drawn to represent different colored balls as atoms or particles and a box to show their composition and movement.
3. What is the particle model?
The particle theory of matter is a model that describes how atoms in a substance are arranged and move. This model explains solids, liquids, and gases’ physicochemical characteristics.
We hope you enjoyed studying this lesson and learned something cool about the Particle Diagram! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
]]>