Halogen Gas Study Guide
INTRODUCTION
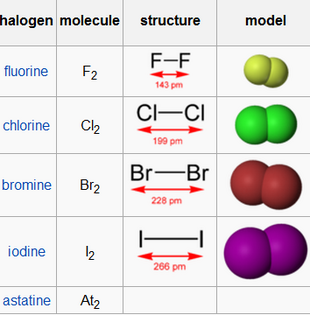
All the elements that belong to the periodic table’s group VIIA (17) are called halogens. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metallic reactive elements that react with hydrogen to generate strong acidic compounds, out of which simple salts can be formed.
The word halogen comes from the Greek origin (hal-, meaning “salt,” and -gen, meaning “to produce.”). All the halogen elements produce sodium salts with similar properties, the most common of which is sodium chloride (NaCl) or table salt, also known as halite.
OXIDATION PROPERTIES OF HALOGEN
One of the few definitions of oxidation in chemistry is the property under which any element removes or takes electrons from any other atom or ion. The electronic configuration of all the elements in the halogen group is that they have a shortage of one electron in their outermost shell.
They can hold the low electron in their p orbital and would ultimately reach the stable electronic configuration. Since they have a great affinity towards taking electrons from different atoms or ions, they are good oxidizing agents. In the process of oxidizing another element, the halogens themselves get reduced. Their oxidation state reduces from 0 to -1. Fluorides, chlorides, bromides,and iodides, are all halides generated when the halogens interact with other elements.
PHYSICAL STATE AT ROOM TEMPERATURE
The physical state of the halogens at room temperature can be explained based on atomic size. The elements of Group 17, when arranged from top to bottom, are in the following order: F, Cl, Br, I, Ar. The atomic size of the elements is in the order I > Br > Cl. This means iodine has more electrons in its outermost shell than bromine, and so does bromine than chlorine.
When the halogen elements exist freely as diatomic molecules, the size order is Iodine > Bromine > Chlorine. As a result, the dispersion force, as well as the intermolecular force, is more in iodine than in bromine than in chlorine. Therefore, the heat required to break these molecules is most for iodine and least for chlorine.
Thus, iodine is solid, and chlorine is gas at room temperature. Bromine at room temperature is liquid.
FAQs
1. Is chlorine a liquid or gas at room temperature?
The state of chlorine at room temperature is gas.
2. Why is chlorine gas at room temperature?
The intermolecular force of chlorine is weak, and the heat required to break the force is less than iodine and bromine at room temperature. That is why Chlorine is a gas at room temperature.
3. At what temperature does chlorine freeze?
Chlorine freezes at -101.5 degrees Celsius or -149.76 degrees Fahrenheit.
4. Mention some of the uses of halogens.
The use of halogens in society is widespread. Table salt or sodium chloride is the most common element required for cooking purposes. We can find fluorides in our daily use products like toothpaste, public water, vitamin supplements, etc. Iodine helps in the functioning of the body’s thyroid glands. Chlorine is used as a water purifier.
We hope you enjoyed studying this lesson and learned something cool about Halogen Gases: Importance & Examples! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Group 17- The Halogens: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_17%3A_The_Halogens#:~:text=The%20halogens%20are%20located%20on,%2C%20and%20astatine%20(At).Accessed 7th March 2022.
- Properties of the Halogens: https://courses.lumenlearning.com/introchem/chapter/properties-of-the-halogens/. Accessed 7th March 2022.