Mendeleev’s Periodic Table Study Guide
INTRODUCTION
Imagine you are in a room with clothes lying on the floor, on the chair; in short, all you can see are clothes everywhere. Won’t you feel anxious in this huge mess of clothes?Imagine what the scientists would have felt when there were too many elements! Let’s see how they managed to sort out the huge number of elements. Let’s take a crash course about the periodic table.
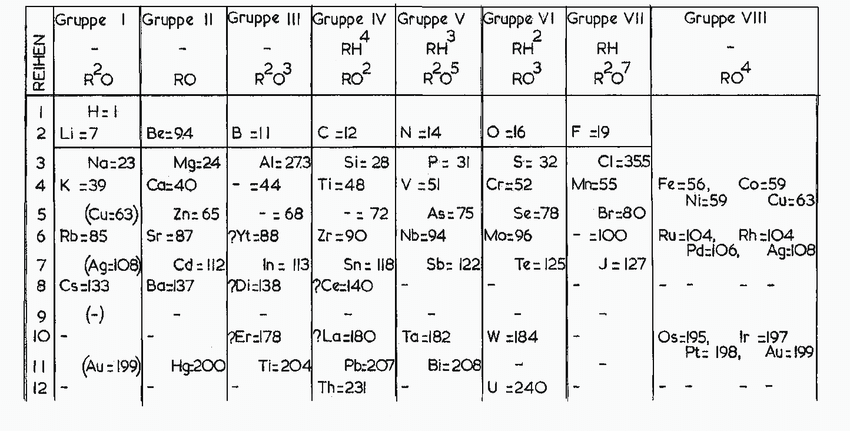
MENDELEEV’S PERIODIC TABLE:
In 1869, a Russian chemist Dmitri Mendeleev thought of organizing the then known 63 elements. He arranged elements based on atomic mass and their chemical properties. Mendeleev realized that atomic mass and properties of elements were interlinked. So, he took 63 cards and wrote one element on each one of them along with the atomic mass and properties known at that time. He arranged the cards in various sequences but found out that the properties repeated when he arranged according to increased masses of elements.
THE DIVISION OF GROUPS:
The groups in the table were divided on the basis of H and O required by the elements to form compounds. For example, in group Ⅰ, hydrogen (H), lithium (Li), sodium (Na) and rubidium (Rb) were placed together as they formed oxides with the same chemical formula. While group Ⅶ elements such as fluorine(F), chlorine(Cl), bromine(I) and iodine(I) were placed together as they form compounds with Hydrogen, i.e. hydrides with the same chemical formula.
THE MISSING ELEMENTS:
The main goal of Mendeleev was to group together elements in ‘groups’ by arranging them according to their atomic masses and properties. But the primary aim was to arrange in order of similar properties. He made gaps for elements that were undiscovered at that time and predicted their properties too. He had made a gap under the element aluminum and named it eka-aluminum, and the element under silicon was named eka-silicon. After several years, gallium was discovered, which took the place of eka-aluminum, and germanium took the place of eka-silicon. The interesting fact is that the properties predicted by Mendeleev were quite similar to the actual properties of elements: Gallium and Germanium.
MERITS OF MENDELEEV’S PERIODIC TABLE:
The gaps that he had left were fitted exactly by the new elements that were discovered in the later years. Also, the new elements could be fitted in the periodic table proposed by Mendeleev without disturbing the place of existing elements.
DEMERITS OF MENDELEEV’S PERIODIC TABLE:
- The position of Hydrogen could not be decided as its properties were similar to group Ⅰ as well as group Ⅶ elements.
- Isotopes of elements that were discovered later couldn’t fit in Mendeleev’s periodic table.
- He didn’t give the total number of elements that were to be predicted.
CONCLUSION:
- Mendeleev had proposed a periodic table based on the mass number and properties of elements.
- Elements with similar properties were placed in the same group and arranged to increase atomic masses.
- He predicted some elements that were yet to be discovered and their properties too. He failed to assign the position of the hydrogen atom, and the isotopes discovered later couldn’t fit in the periodic table.
FAQs:
1. What was the basis of Mendeleev’s periodic table?
Elements were arranged together with similar properties and in order of increasing atomic masses.
2. Eka- aluminum, as predicted by Mendeleev turned out to be which element?
Gallium, which was discovered later, had properties similar to that predicted for Eka- aluminum.
3. What were the demerits of Mendeleev’s periodic table?
He failed to assign the position of the hydrogen atom, and also, the isotopes which were discovered later couldn’t fit in the periodic table.
We hope you enjoyed studying this lesson and learned something cool about Mendeleev’s Periodic Table! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎.
SOURCES:
- The Mendeleev’s Periodic Table: https://www.researchgate.net/figure/The-Mendeleev-Periodic-Table-of-1871_fig1_323028562. Accessed 25 Feb 2022.
- Periodic Classification of Elements: https://www.tetsuccesskey.com/2015/08/periodic-classification-of-elements.html. Accessed 25 Feb 2022.