Enthalpy Study Guide
INTRODUCTION
You might have heard that you have to work out and be physically active to burn fat or calories. Have you ever wondered what this “burning” is exactly? Where does this take place? It’s an analogy that is often used. There is no smoldering fire 🔥 inside our bodies. When we work out 💪, we often say that we burn calories. In reality, the food goes through a series of oxidation processes and is converted into products like CO₂ and H2₂.
These oxidation processes release the energy we need for our day-to-day activities. And the more active we are, the more energy will be required, and the more calories will be burned by our bodies to keep up with the demand. In thermodynamics, enthalpy is a thermodynamic property that helps us establish this heat-energy relationship in real life.
ENTHALPY
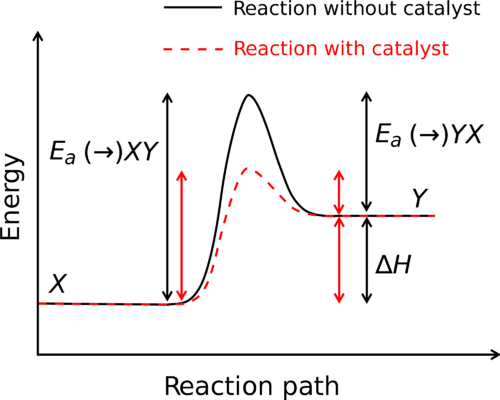
A system’s enthalpy is a thermodynamic attribute. It’s the product of the system’s pressure and volume multiplied by internal energy. It indicates the ability to perform non-mechanical labor and the ability to dissipate heat. H stands for enthalpy, while h stands for specific enthalpy. The Joule and Calorie are standard units for expressing enthalpy. In a throttling process, enthalpy remains constant.
Since the total enthalpy of a system could not be determined because the zero point cannot be known, the change in enthalpy is estimated instead of enthalpy. On the other hand, the variation in enthalpy between two states may be measured. Under constant pressure, the change in enthalpy may be determined.
ENTHALPY FORMULA
H = E + PV where,
H – Enthalpy
E – Internal energy of the system
P- pressure
V – volume
IMPORTANCE OF ENTHALPY
We can determine if a reaction was endothermic or exothermic by monitoring the difference in enthalpy.
- It’s used to figure out how much heat a chemical reaction produces.
- In calorimetry, the change in enthalpy is used to quantify heat flow.
- A compressor’s lowest power is calculated using enthalpy.
- During a transition from one state of matter to another, enthalpy changes.
- Enthalpy has several more uses in thermal engineering.
CONCLUSION
- The heat component of a system under constant pressure is called enthalpy.
- Because most chemical reactions occur at constant pressure, enthalpy is employed to calculate the temperatures of the reaction rather than internal energy.
- At constant pressure, the heat equals the system’s enthalpy change.
FAQs:
1. What is enthalpy in simple terms?
The evaluation of energies in a thermodynamic system is called enthalpy. The overall content of heat in a system is enthalpy, which is equal to the system’s internal energy plus the product of pressure and volume.
2. What are enthalpy and entropy?
Entropy is the degree of inherent disorder within a substance. In contrast, enthalpy is the degree of internal energy stored in the complex. The degree of disorder, or entropy, is usually highest in gasses and least in solids.
3. How is enthalpy calculated?
The enthalpy, H, is equal to the sum of the system’s internal energy, E, and the combination of its pressure, P, and volume, V: H = E + PV. As per the rule of energy conservation, the energy change is equivalent to the energy transmitted to the system less the work done by it.
4. Is enthalpy the same as heat?
Enthalpy is the quantity of heat transmitted throughout a chemical reaction under constant pressure, whilst heat is a type of energy. Heat and enthalpy are two concepts that describe a system’s energy flow and attributes.
We hope you enjoyed studying this lesson and learned something cool about Enthalpy! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Internal Energy and Enthalpy. https://courses.lumenlearning.com/introchem/chapter/internal-energy-and-enthalpy/. Accessed 18 February 2022
- Enthalpy .https://www.ck12.org/c/chemistry/enthalpy/lesson/Enthalpy-CHEM/?referrer=concept_details. Accessed 18 February 2022
- Enthalpy Definition in Chemistry and Physics. https://www.thoughtco.com/definition-of-enthalpy-605091. Accessed 18 February 2022