Electrolytes And Non-Electrolytes Study Guide
INTRODUCTION
Depending on whether chemical compounds conduct electricity through their aqueous solution, they can be divided into two distinct parts, including electrolytes and non-electrolytes. Chemistry definition of electrolyte states that it is a chemical compound that could easily dissolve in an aqueous solution (water), creating ions.
The formed ions are the prime source behind conducting electricity through the solution. Non-electrolytes do not conduct any electricity when dissolved in water because they cannot form any ions.
ELECTROLYTE IN CHEMISTRY
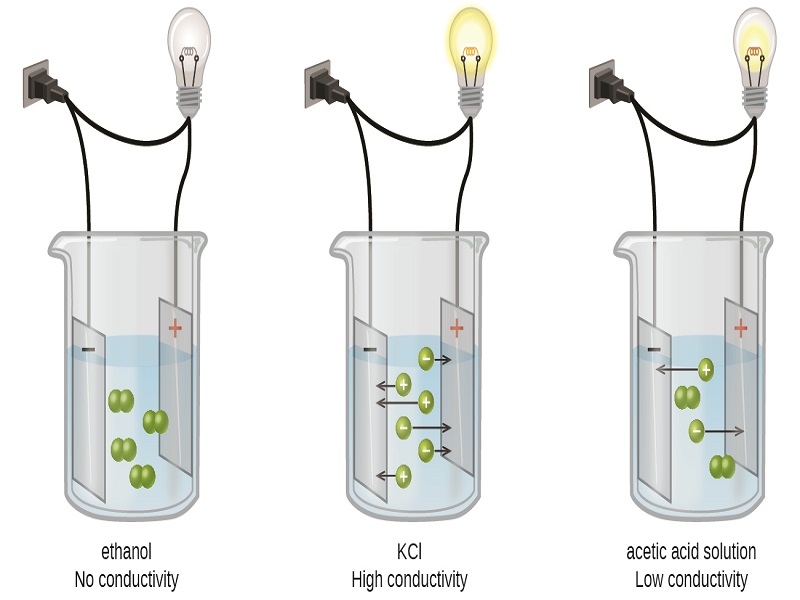
When dissolved in water, electrolytes are those chemical compounds that could break down into ions and conduct electricity throughout the aqueous solution. However, the compound should be an ionic compound made of anions and cations to break down into ions. The ions break down when you dissolve electrolyte compounds in the aqueous solution, forming aqueous cations and anions dispersed uniformly.
The solution is neutral due to the uniform distribution of ions. You must have seen this experiment where an electric current is introduced into the aqueous solution, and the ions start moving to the two ends. This experiment helps prove electrolytes conduct electricity.
However, electrolytes can again be divided into weak and strong electrolytes. You would not find any neutral molecules in the aqueous solution of a strong electrolyte as it completely ionizes. Although, with weak electrolytes, the ions do not get completely ionized; therefore, you might find some neutral molecules there.
EXAMPLES OF ELECTROLYTES
Strong bases and acids are great examples of electrolytes as they get completely ionized in water. However, some compounds only dissolve partially but are still considered strong electrolytes like strontium hydroxide.
There are some weak electrolytes as well, like mostly nitrogen-containing compounds. Water is a strong example of a weak electrolyte.
NON-ELECTROLYTE COMPOUNDS
These chemical compounds cannot conduct electricity. Most non-electrolyte compounds are covalent compounds; they do not form any ion. Hydrocarbons are primary examples of non-electrolytes because they cannot dissolve in water.
CONCLUSION
- Electrolytes could break into ions and can conduct electricity
- Non-electrolytes do not get dissolved in water; therefore, they cannot conduct electricity
- Strong acids alongside bases are good examples of electrolytes.
- Hydrocarbons are examples of non-electrolytes.
FAQs:
1. What are electrolytes and their types?
Electrolytes are chemical compounds that can conduct electricity. The two types are strong electrolytes and weak electrolytes.
2. How do you identify an electrolyte in chemistry?
If a chemical compound dissolves in water and then separates into cations and anions, it is an electrolyte, and if it fails to do so, it is a non-electrolyte.
We hope you enjoyed studying this lesson and learned something cool about Electrolytes and Non-electrolytes! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Difference between Electrolytes and Non-electrolytes: https://pediaa.com/difference-between-electrolytes-and-nonelectrolytes/#:~:text=Electrolytes%20are%20chemical%20compounds%20that,ions%20when%20dissolved%20in%20water. Accessed 28 Feb 2022.
- Non-electrolyte: https://byjus.com/chemistry/nonelectrolyte/#:~:text=Examples%20of%20Nonelectrolytes&text=Glucose%2C%20a%20sugar%20with%20the,it%20is%20considered%20a%20nonelectrolyte. Accessed 28 Feb 2022.