Wave-Particle Theory Study Guide
INTRODUCTION
In the mornings, it’s always lovely to see a ray of sunlight gleaming through our windows. But have you ever considered what light is and how it travels from the sun to us? Well, this was a significant question in the history of science, and the solution includes the names of some of the world’s most brilliant thinkers. Let’s look at this in more detail.
THE HISTORY OF THE WAVE-PARTICLE THEORY
Christiaan Huygens and Isaac Newton offered opposing ideas about the behavior of light in the 1600s. Huygens and Newton proposed a wave theory and particle theory, respectively. But Newton’s fame aided in the acceptance of his theory, and for more than a century, Newton’s hypothesis reigned supreme.
Complications occurred for the particle theory of light in the early nineteenth century. Thomas Young’s double-slit experiment revealed clear wave activity, implying that the wave theory of light prevailed over Newton’s particle theory.
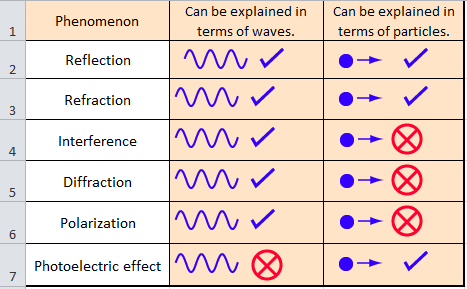
It was in 1905 that Albert Einstein hypothesized that light travels as discrete bundles of energy in his article explaining the photoelectric effect.
THE WAVE-PARTICLE THEORY
The de Broglie hypothesis explored the question of whether such duality manifested itself in the matter. He stated that for a particle of momentum mv, the wavelength is given by ℷ=h/mv.
Matter, like light, appeared to have both wave and particle qualities when the conditions were correct. The wave-particle theory definition states that in Quantum theory, matter and light are made up of minute particles having wave characteristics.
The key difference between the wave and particle theory is that entities like electrons and photons, according to particle theory, are minuscule objects that do not spread beyond a narrow volume, whereas electrons and photons are packets of field energy, as per the wave theory.
EINSTEIN’S LIGHT QUANTUM HYPOTHESIS
- In 1905, Einstein was capable of explaining the photoelectric effect, which had previously been unfathomable when the light was viewed as a wave.
- Light, according to Einstein, is a particle whose wavelength corresponds to its energy.
- To grasp this phenomenon, consider photons as energy-containing (clusters of) particles.
- Particles with high energy and the ability to emit electrons are referred to as blue light.
- Particles with low energy that are unable to emit electrons produce red light.
- Because light possesses the attributes of both a wave and a particle, it was given the name “photons (light quanta)” in this fashion.
SUMMARY
- In 1905 Albert Einstein hypothesized that light travels as discrete bundles of energy in his article explaining the photoelectric effect.
- Matter, like light, appears to have both wave and particle qualities when the conditions are correct.
- For a particle of momentum mv, the wavelength is given by ℷ=h/mv.
- Because light possesses the attributes of both a wave and a particle, it was given the name “photons (light quanta)” in this fashion.
FAQs
Q. What is the wave-particle duality theory?
the wave-particle duality theory is a Quantum theory that states that matter and light are made up of minute particles having wave characteristics
Q. Who proposed the wave-particle theory?
Albert Einstein proposed the wave-particle theory.
Q. Which process describes the particle nature of light?
The photoelectric effect describes the particle nature of light.
We hope you enjoyed studying this lesson and learned something cool about Wave-Particle Theory! Join our Discord community to get any questions you may have answered and to engage with other students just like you! We promise, it makes studying much more fun!😎
REFERENCE
- Wave-Particle Theory: https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/18.4/primary/lesson/wave-particle-theory-ms-ps/ Accessed 14th April 2022.