Quantum Mechanical Atomic Model Study Guide
INTRODUCTION
In 1926, Schrodinger used the duality of electron wave particles to create and solve difficult mathematical formulas that accurately describe the behavior of electrons in a hydrogen atom. The quantum mechanical model of the atom was created by solving the Schrodinger equation.
Quantum mechanics models describe the possibility of placing electrons within an atom by describing the main energy level, orbital (arbitrary level), and spin.
FEATURES OF QUANTUM MECHANICAL MODEL OF ATOM
-
The power of the electron is quantized. That is, it can have a positive performance value.
-
The proven answer to the Schrodinger wave equation is the quantized power of the electron, which is the end result of the wave-like properties of the electron.
-
According to Heisenberg’s Uncertainty Principle, it is not possible to know the specific function and momentum of an electron. Therefore, we can calculate the only danger of putting an electron in a particular function.
-
The current number of miles is | ψ | 2. Where ψ represents the wave characteristics of the electron.
-
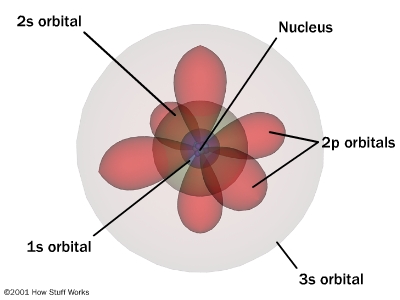
The wave characteristics (ψ) of electrons in an atom are called atomic orbitals. Electrons occupy atomic orbitals when their miles are characterized by wave characteristics. Since an electron can have many wave functions, it has multiple atomic orbitals.
-
Each wave’s characteristics or atomic orbitals have a specific shape and power.
-
The orbital wave properties of an atom contain all the statistics about the electrons in the atom, and quantum mechanics can extract these statistics.
-
The likelihood of an electron being placed on a positive web page within an atom is proportional to the square of the orbital wave characteristic at that location. That is, | ψ | 2. Random density is always advantageous and is understood as | ψ | 2.
PRINCIPLES OF QUANTUM THEORY
According to quantum theory, an electron can only exist in a limited number of quantized energy levels. It also teaches us that no two electrons in the same system can be in the same energy state at the same time and that all energy states are filled from the lowest to the highest levels.
QUANTUM MECHANICAL MODEL OF ATOM STRUCTURE
Quantum mechanical models of atoms use the complex shape of orbits (also known as electron clouds), which are the volumes of space in which electrons are likely to be present. As a result, this model is based on probability rather than certainty.
DRAWBACKS OF THE QUANTUM MECHANICAL MODEL OF THE ATOM
The chance of finding an electron at a given distance from the nucleus is calculated using the quantum mechanical model of an atom. The graphs are difficult to understand since they are wave functions rather than basic distance vs probability plots. Quantum mechanics is not to be comprehended and is not comprehended by humans.
SUMMARY
- Quantum mechanics models describe the possibility of placing electrons within an atom by describing the principal energy level, energy level, orbital (arbitrary level), and spin of.
- The quantum mechanics model is based on quantum theory, which states that matter has wave-like properties.
- According to quantum theory, it is impossible to know the exact position and momentum of an electron at the same time.
FAQs
Q. What is the quantum mechanical model? Explain.
Quantum mechanics models describe the possibility of placing electrons within an atom by describing the principal energy level, energy level, orbital (arbitrary level), and spin of.
Q. What is the quantum mechanical model of the atom based on?
Schrödinger’s wave equation and its solution are the foundations of quantum mechanics. The idea of shells, subshells, and orbitals emerges from the solution of the wave equation.
Q. What are the main points of the quantum mechanical model?
(i) Quantization of electron energy in atoms.
(ii) Quantized electronic energy levels exist as a direct outcome of electrons’ wave-like characteristics.
(iii) An electron’s exact position and velocity in an atom cannot be known at the same time (Heisenberg uncertainty principle).
We hope you enjoyed studying this lesson and learned something cool about Quantum Mechanical Atomic Model! Join our Discord community to get any questions you may have answered and to engage with other students just like you! We promise, it makes studying much more fun!😎
REFERENCE
- Quantum Mechanical Model Simplified: https://byjus.com/chemistry/quantum-mechanical-model-simplified/ Accessed 19th April 2022.
- Quantum Mechanical Atomic Model: https://www.geeksforgeeks.org/quantum-mechanical-atomic-model/#:~:text=Schr%C3%B6dinger%20used%20the%20electron’s%20wave,mechanical%20model%20of%20the%20atom. Accessed 19th April 2022.
- Quantum Mechanical Model: https://www.toppr.com/ask/content/concept/quantum-mechanical-model-202814/ Accessed 19th April 2022.
- The Quantum Mechanical Model of Atom: https://www.khanacademy.org/science/physics/quantum-physics/quantum-numbers-and-orbitals/a/the-quantum-mechanical-model-of-the-atom Accessed 19th April 2022.