Change Of State Study Guide
INTRODUCTION
It’s our duty to protect our environment, and metal recycling is one of the numerous ways we can contribute to the preservation of our lovely environment. Recycling metals decreases ore mining waste by 97%, according to statistics. But, how do you believe this occurs? It’s simple: the recycled metal is collected and placed in a furnace, where it is melted into new metal sheets. These alterations are referred to in science as a change in the state of matter.
DIFFERENT STATES OF MATTER
- It has been discovered that matter exists in various forms throughout nature.
- Some things are stiff and have a fixed shape; others, such as water, can flow and take the shape of their container; and still others, such as air, have no defined shape or size.
- Based on its physical properties, matter can be classified into three types of states of matter – Solid, Liquid, and Gas.
CHANGING OF THE STATE
-
Change of state means that there is a physical change in matter.
-
When matter loses or absorbs energy, this happens.
-
For instance, when a body absorbs energy, its atoms and molecules move faster, and the extra kinetic energy forces particles far enough away from each other that they change shape.
-
Temperature and pressure changes are the two main reasons for the phase change.
-
The contact between molecules increases as the temperature or pressure rises.
-
Likewise, as the temperature drops, molecules and atoms find it easier to form a more rigid structure.
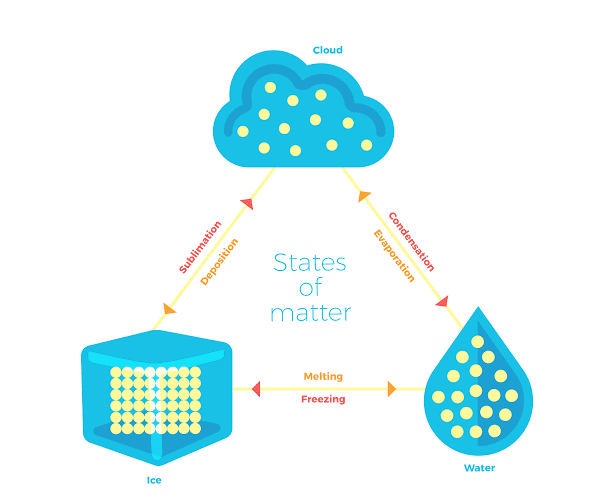
PROCESSES INVOLVED IN THE CHANGE OF STATE
Melting, freezing, sublimation, deposition, condensation, and vaporization are the various processes that cause a change between various states. Below is the change in the state diagram.
-
Melting is the transformation of a solid into a liquid state. The melting point of a solid is the temperature at which it transforms into a liquid.
-
Freezing is the phenomenon of liquid water turning into solid ice. The freezing point is the temperature at which it happens.
-
Condensation is the process through which gas transforms into a liquid.
-
Vaporization is the mechanism through which a liquid boils and transforms into a gas. The boiling point of a liquid is the temperature at which it boils and starts transforming into vapor.
-
Sublimation is the process of solids converting straight to gasses. Sublimation occurs when solids, such as dry ice are heated.
SUMMARY
- Solid, Liquid, and Gas are the three different states of matter.
- Changes in state are physical changes in matter.
- Melting, freezing, sublimation, deposition, condensation, and vaporization are the various processes involved in the change of state.
FAQs
Q What is the boiling point?
The boiling point of a liquid is the temperature at which it boils and starts transforming into vapor.
Q. What is the melting point?
The melting point of a solid is the temperature at which it transforms into a liquid.
Q. What is the process in which solids directly transform into a gas?
Sublimation is the process of solids converting straight to gasses.
Q. What is evaporation?
Evaporation is the process of liquids turning into gas.
We hope you enjoyed studying this lesson and learned something cool about the Change of State! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
REFERENCE
- Change of State: https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/2.17/primary/lesson/changes-of-state-ms-ps/. Accessed 13th April 2022.
- State of Matter: https://en.wikipedia.org/wiki/State_of_matterAccessed 13th April 2022.