Tonicity and Osmoregulation Study Guide
Introduction
Water moves from high concentration to low concentration, just like other substances. The movement of water inside our bodies is essential for survival. Water moving into the cells or outside leads to many consequences. Let’s study what factors and how they affect water’s movement in or around the cells.
Tonicity
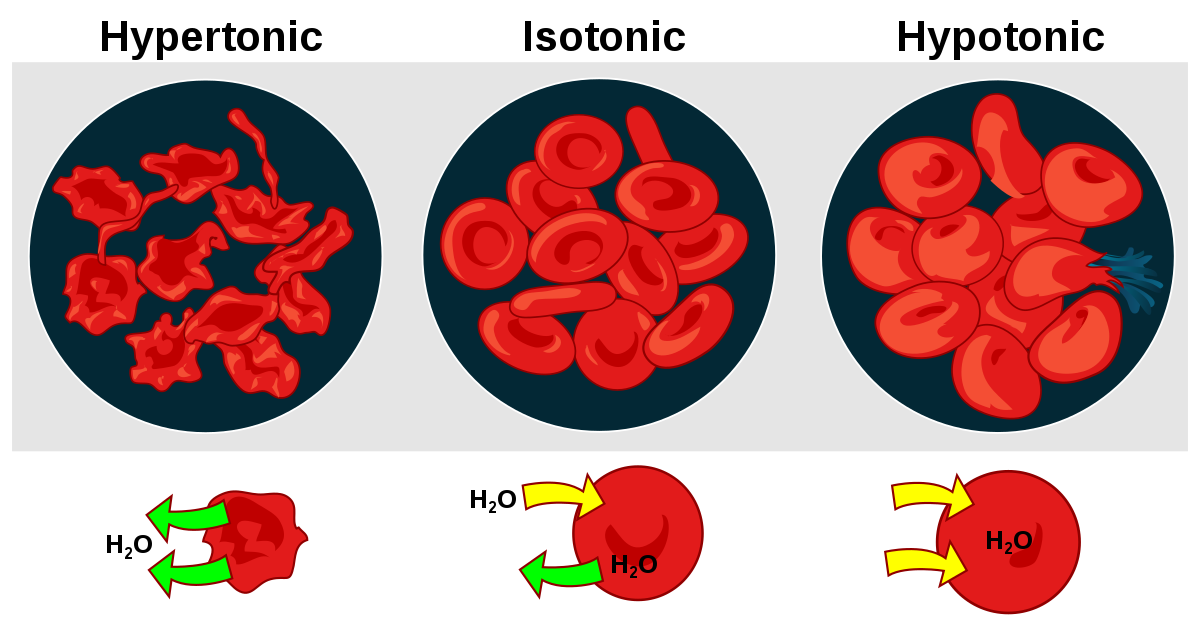
Tonicity is the ability of an extracellular solution to make water move into or out of a cell through osmosis. Tonicity depends upon relative solute concentrations and the cell membrane’s permeability to those solutes. There are three terms (hypertonic, hypotonic, isotonic) used to determine tonicity. They describe whether a solution will cause the water to move into or out of a cell.
-
Hypertonic- A solution is described as a hypertonic solution to a cell if its solute concentration is higher than inside the cell. Thus, the solute is unable to cross the membrane and stays outside. Hence the net flow of the water too is outwards, where there is more solute, causing the cell to lose volume and shrink.
-
Hypotonic- A solution is described as a hypotonic solution to the cell if the solute concentration outside the cell is lower than inside the cell, hence the flow of water is into the cell, where there is more solute, causing the cell to gain volume and expand.
-
Isotonic- A solution is described as isotonic if the solute concentration outside and inside the cell is the same. Hence there is no water flow inside out of the cell, keeping the cell volume the same.
Concentration Gradient
The movement of molecules across membranes is affected by the concentration gradient. A concentration gradient occurs when the concentration of particles is higher in one area than another. In passive transport, particles will diffuse down a concentration gradient, from areas of higher concentration to areas of lower concentration, until they are evenly spaced.
Tonicity in Livings
In red blood cells
- If red blood cells are placed in a hypotonic solution, RBCs will bloat up and may also explode.
- RBCs will shrivel if red blood cells are placed in a hypertonic solution, making their content concentrated.
- Only isotonic conditions are ideal for survival.
Plant cells
- If a plant is not watered, the extracellular fluid becomes isotonic or hypertonic, leading the water to leave the plant’s cell.
- It causes loss of turgor pressure, leading to wilting.
- In the case of plant cells, only a hypotonic extracellular solution is regarded as ideal.
Osmosis and Osmolarity
Osmosis is the net movement of water across a semipermeable membrane from a lower solute concentration area to a higher solute concentration area.
Osmolarity tells us about the total concentration of the solutes in a solution. When solutions of different osmolarities are separated by a permeable membrane (permeable to water only, not to solute), water will move from the lower osmolarity side to the higher osmolarity side.
- Low Osmolarity- Fewer solute particles per liter of the solution.
- High Osmolarity- More solute particles per liter of the solution.
- Osmosis plays an important role in the water balance of the cells.
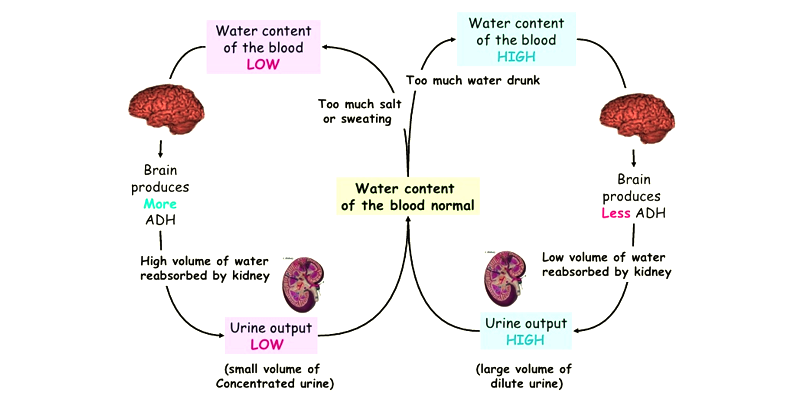
Osmoregulation
Osmoregulation is defined as how living things regulate the effects of osmosis to protect cellular integrity. It refers to the physiological processes that maintain a fixed concentration of cell membrane-impermeable molecules and ions in the fluid that surrounds cells.
Mechanism of osmoregulation involves:
- Multiple bodies to brain signaling mechanisms.
- A brain neural network
- Reflex- Autonomic and endocrine
- Behavioral mechanisms
Importance of osmoregulation
Osmoregulatory mechanisms contribute to the health and survival of organisms.
- Osmoregulation controls the blood’s water levels and salt (mineral ions). The excess salt or water is expelled out (with the help of special organs), and the right concentration is always maintained.
- A marine fish has an internal osmotic concentration lower than that of the surrounding seawater, so it tends to lose water and gain salt. It actively excretes salt out from the gills.
- Kidneys play a big role in osmoregulation in humans by regulating the osmotic pressure of human’s (mammals) blood through extensive filtration and purification.
Conclusion
- Tonicity and osmoregulation play a crucial part in all living beings’ survival.
- Osmoregulation maintains water balance and allows organisms to control their internal solute composition/water potential. Osmoregulation is an example of homeostasis.
- Osmosis plays an important part in many biological processes.
FAQs:
1. What role does tonicity play in osmoregulation?
Tonicity is the ability of a surrounding solution to cause a cell to gain or lose water. It plays an important part in osmoregulation as tonicity decides whether a cell can maintain water balance or not.
2. What is the difference between tonicity and osmosis?
Osmosis is defined as the number of solutes dissolved in a volume of solution. Tonicity is a comparison of a solution and a cell. Osmosis has units, whereas tonicity has no units.
3. What is a tonicity example?
A very common example of tonicity is freshwater vs. saltwater beings. For example, saltwater fish cannot live in fresh water and vice-versa. If we put a saltwater fish in freshwater, due to low osmolarity, water will flow into the cells of the fish, eventually causing them to burst and killing the fish.
4. What is tonicity, and what does it depend on?
Tonicity is defined as the ability of a solution which surrounds the cell to cause the cell to gain or lose water. It depends on the relative concentration of solutes across a cell membrane which determines the direction and extent of osmotic flux. Three terms are used to determine tonicity- hypertonic, hypotonic, isotonic. They describe whether a solution will cause the water to move into or out of a cell.
5. What is the importance of tonicity?
Tonicity is very important to understand as it gives a good insight into how dehydration can affect the body’s cells. Tonicity plays a crucial part in fluid balance. It is critical in the regulation of the volume of body cells. Disturbances in tonicity result from abnormalities in the relation between body water and body solute. The concept of extracellular volume plays a critical role in regulating the perfusion of body cells and organs.
*We hope you enjoyed studying this lesson and learned something cool about Tonicity and Osmoregulation! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! *😎
Sources:
- Osmoregulation. https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/osmoregulation. Accessed 17 Dec, 2021.
- Homeostasis and Osmoregulation. https://opentextbc.ca/biology/chapter/11-1-homeostasis-and-osmoregulation/. Accessed 17 Dec, 2021.
- A brief history of the study of fish osmoregulation: the central role of the Mt. Desert Island Biological Laboratory. https://www.frontiersin.org/articles/10.3389/fphys.2010.00013/full. Accessed 17 Dec, 2021.
- Tonicity and Osmoregulation https://apcentral.collegeboard.org/pdf/ap-biology-course-and-exam-description-0.pdf?course=ap-biology Accessed 17 Dec, 2021.