CBSE Class 11 Biology Chapter 12 Revision Notes
Chapter 12: Mineral Nutrition Revision Notes
Multiple Choice Questions
- Which of the following is a constituent of cell membranes and certain proteins?
- The main constituent in ferredoxin is _______.
- Decomposition of organic nitrogen of dead plants and animals into ammonia is called________.
- Which of the following is NOT a nitrogen-fixing bacteria?
- Identify the element that plays an important role in the opening and closing of stomata.
- The deficiency of which of the following elements is NOT likely to cause delayed flowering if their concentration in plants is low?
- Which of the following is a prominent symptom of manganese toxicity?
How are the mineral requirements of plants identified?
Hydroponics
- Hydroponics is a method of growing plants in a nutrient solution without soil.
- This approach is used to determine which nutrients are necessary for plants to grow.
- The plant is cultivated in a soil-free, specified mineral solution in this procedure.
- Purified water and mineral fertilizers are required for these approaches.
- Hydroponics procedures are used to identify essential components and associated deficiency symptoms.
- It’s also utilized for commercial vegetable cultivation, such as tomato and cucumber.
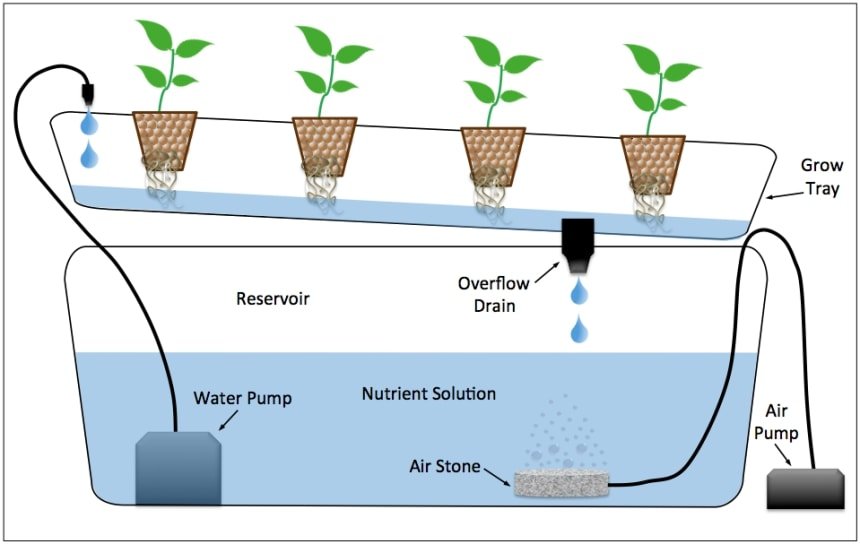
Source: Hydroponics
How do you know if a certain nutrient is essential for plant growth?
The following are the factors for determining whether or not an element is essential
(a) The element must be absolutely required for normal development and reproduction to occur. Plants do not finish their life cycle or set seeds in the absence of the element.
(b) The element’s need must be unique and cannot be replaced by another element. In other words, a deficit of one element cannot be compensated by adding another.
(c) The element must play a direct role in the plant’s metabolism.
Plant Nutrients
Plant nutrients are divided into two categories: Macronutrients and micronutrients.
- Macronutrients are found in greater abundance in plant tissues.
- Water and carbon dioxide provides C, H, and O, while the remainder is absorbed from the soil.
- Examples include carbon, hydrogen, oxygen, nitrogen, phosphorous, sulfur, potassium, calcium and magnesium.
- Micronutrients, often known as trace elements, are only necessary in tiny amounts.
- Examples include iron, manganese, copper, molybdenum, zinc, boron, chlorine and nickel
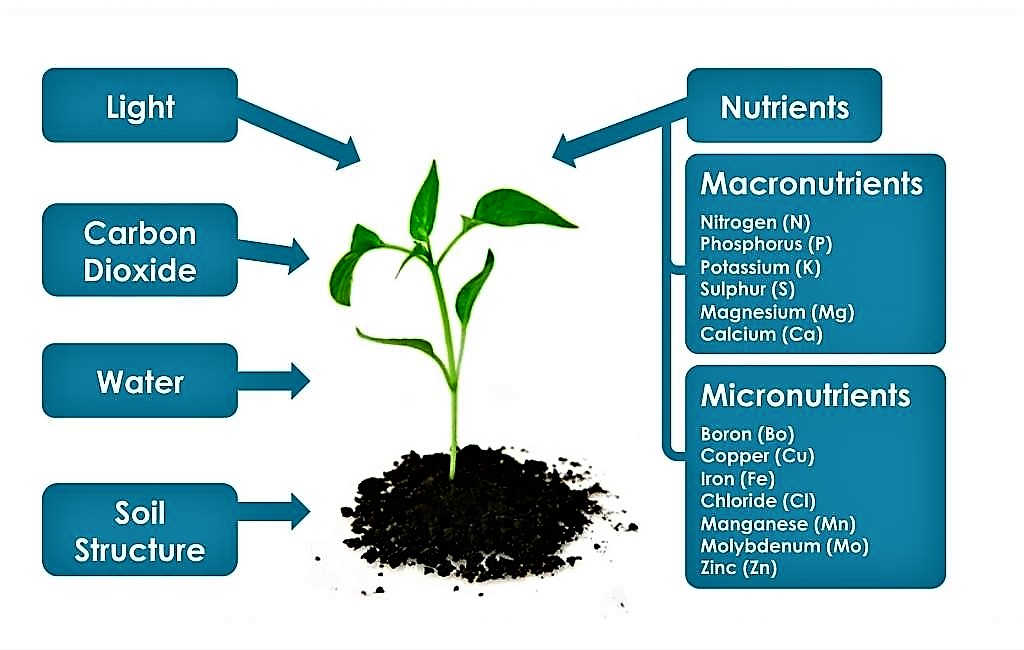
Source: Plant nutrients
Role of Macro- and Micro-nutrients
Nitrogen
- It is the most abundant element in plants, and they absorb it as NO2–, NO3–, and NH4.
- Found mostly in the meristematic tissues and the metabolically active cells.
- The conversion of nitrogen as occurring naturally into ammonia is termed **nitrogen fixation.
Phosphorus
- Plants absorb phosphate ions from the soil.
- It is a component of the cell membrane, nucleic acids, nucleotides, proteins and phosphorylation reactions.
Potassium
- Potassium ions (K) assist in the maintenance of the cation-anion equilibrium in cells.
- Protein synthesis and the opening and shutting of stomata.
- Activation of enzymes and cell turgidity.
Calcium
- Calcium ions (Ca2+) are absorbed by plants from the soil and found in the meristematic and differentiating tissues.
- It’s used to make cell walls (calcium pectate), mitotic spindle, metabolic activities and certain enzyme activation.
Magnesium
- Mg2+ divalent ions are absorbed by plants.
- Activation of respiration and photosynthesis enzymes, and DNA and RNA synthesis.
- It’s a component of chlorophyll and the ribosome structure.
Sulfur
- In the form of sulfate (SO42-).
- It’s found in amino acids (cysteine, methionine), as well as coenzymes and vitamins.
Iron
- Ferric iron (Fe3) is the form of iron that is obtained and helps in chlorophyll formation (catalase).
- It’s a crucial component of ferredoxin and cytochromes.
- It is reversibly oxidized from Fe2+ to Fe3+ during electron transfer.
Manganese
- It is absorbed as Mn2 ions and found in photosynthesis, respiration and nitrogen metabolism enzyme activation.
- During photosynthesis, manganese splits water to free oxygen.
Zinc
- It is acquired in the form of Zn2 ions.
- Activates carboxylase enzymes and helps in auxin synthesis.
Copper
- Is absorbed in the form of cupric ions (Cu2+).
- Involved in a variety of metabolic and redox process enzymes (reversibly oxidised from Cu+ to Cu2+).
Boron
- Is absorbed as the ions BO33- and B4O72-.
- Boron is required to uptake and utilize Ca2+, membrane functioning, pollen germination, cell elongation, cell differentiation, and carbohydrate translocation.
Molybdenum
- Molybdate ions are a component of nitrogenase and nitrate reductase (nitrogen metabolism)
Chlorine
- Chloride anion (Cl– ) helps in cell solute concentration and the anion-cation balance.
- It is essential for the water-splitting reaction in photosynthesis
Deficiency of Essential Elements
- The critical concentration of essential elements is a concentration below which plant development is retarded.
- The deficiency of a certain element causes morphological changes and are called deficiency symptoms.
- The mobility of elements in the plants determines which plant areas develop deficiency symptoms.
- Actively mobilized elements (N, Mg, K) indicate a shortage in older locations.
- If the components are generally stationary (Ca) and not moved out of mature tissues, symptoms develop initially in the juvenile region.
- Deficiency of N, K, Mg, S, Fe, Mn, Zn and Mo: Chlorosis
- Deficiency of Ca, Mg, Cu, K: Necrosis
- Lack or low level of N, K, S, Mo: Inhibition of cell division
- Low level of N, S, Mo: Delayed flowering
Micronutrient toxicity
- Micronutrients become harmful in larger amounts (narrow range of optimum concentration).
- Any mineral ion concentration in tissues that reduces the dry weight of tissues by about 10 per cent is considered toxic.
- Manganese toxicity: appearance of brown spots surrounded by chlorotic veins song with inducing deficiencies of iron, magnesium and calcium.
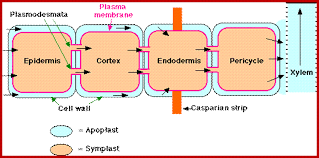
MECHANISM OF ABSORPTION OF ELEMENTS
Absorption of Elements: Mechanism
Source: Absorption of elements mechanism
- Mineral translocation occurs in the xylem via a transpirational pull.
- First phase: rapid ion intake occurs in the open or outside space of the cells, the apoplast. Ion channels form selective pores.
- Second phase: ions are gently absorbed into the interior space of the cells, the symplast. Active method of transport (energy spent).
- Flux is the term commonly used for ion movement.
- Inflow refers to inner movement, whereas efflux refers to outward movement.
SOIL AS RESERVOIR OF ESSENTIAL ELEMENTS
- Weathering and breakdown of rocks provide the necessary ions and inorganic salts.
- Since they are derived from the rock minerals, their role in plant nutrition is referred to as mineral nutrition.
Metabolism of Nitrogen
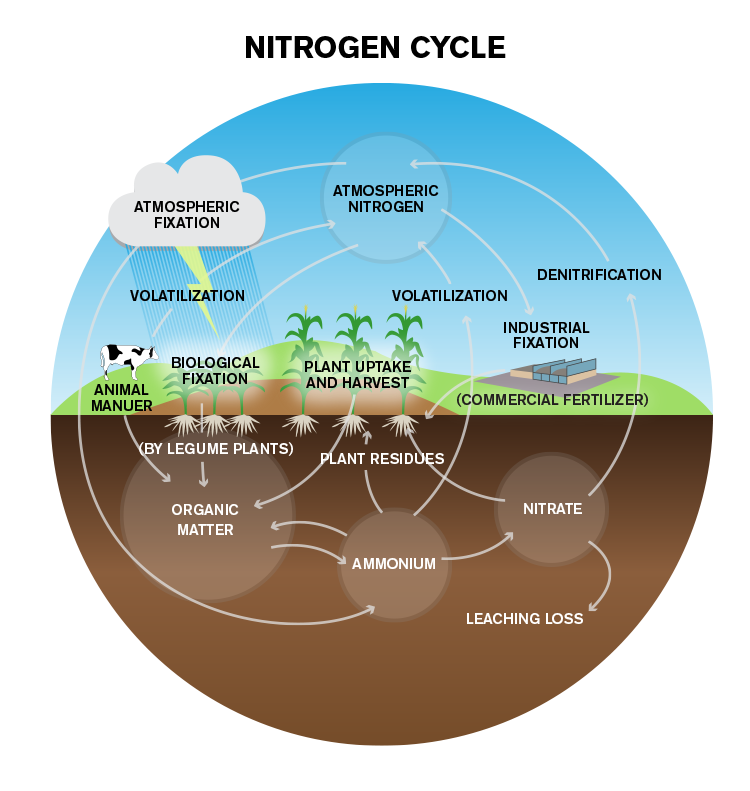
- The biological process of nitrogen fixation is the first step in the nitrogen cycle.
- Nitrogen fixation is the process of conversion of nitrogen (N2 ) to ammonia.
- Decomposition of organic nitrogen of dead plant s and animals into ammonia is called ammonification.
- Ammonia is first oxidized to nitrite by the bacteria Nitrosomonas and/or Nitrococcus.
- The nitrite is further oxidized to nitrate with the help of the bacterium Nitrobacter.
- These steps are called nitrification (nitrifying bacteria are chemoautotrophs).
Biological Nitrogen FIxation
- Plants are unable to use nitrogen from the atmosphere. Some nitrogen-fixing bacteria transform nitrogen from the atmosphere into ammonia (nitrogenase enzyme), which plants may use.
- Reduction of nitrogen to ammonia by living organisms is called biological nitrogen fixation.
- Azotobacter, Bacillus, Anabaena are nitrogen fixers.
- The legume-bacteria symbiotic relationship: Rhizobium with the roots of legumes like sweet pea as nitrogen-fixing nodules
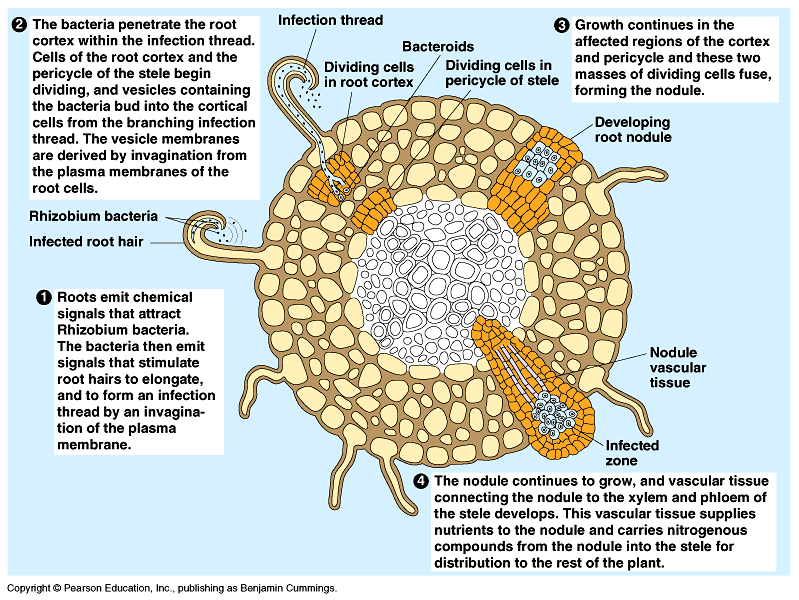
Source (please replace with this new image in the CMS)
- The nodule contains the enzyme nitrogenase (Mo-Fe protein) and leghaemoglobin.
- Nitrogenase requires anaerobic conditions provided by oxygen scavenger called leg-hemoglobin
- Nitrogenase catalyzes the conversion of atmospheric nitrogen to ammonia (8 ATP for each NH3 produced from host cell respiration)
- The ammonia is protonated to form NH4 + (ammonium) ion.
- NH4 + is used to synthesize amino acids in plants in two ways:
-
Reductive amination
Ammonia reacts with α-ketoglutaric acid and forms glutamic acid.
-
Transamination
The transfer of an amino group from one amino acid (Glutamic acid) by transaminase to the keto group of a keto acid.
Asparagine and glutamine found in plant proteins are formed from two amino acids, namely aspartic acid and glutamic acid (hydroxyl group replaced by another amino group).
In soyabean the fixed nitrogen is exported as ureides
]]>