CBSE Class 11 Chemistry Chapter 1 Revision Notes
Chapter 1: Basic Concepts of Chemistry Revision Notes
Chemistry
- It is a discipline of science that studies matter’s composition, structure, and characteristics.
- The father of chemistry is Antoine Laurent Lavoisier.
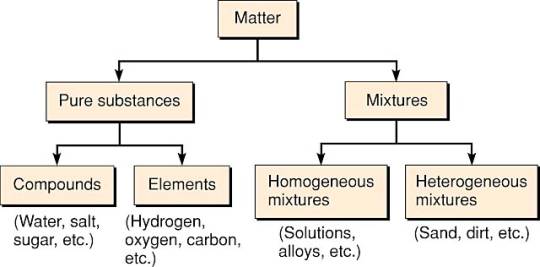
Matter
- Matter is defined as everything that takes up space and has mass.
- It is made up of microscopic particles that are separated by space.
- The matter particles are attracted to one another and are in constant motion.
Elements
- It is the most basic form of pure material, unable of being decomposed into or constructed from simpler compounds using conventional physical and chemical procedures.
- There are just one type of atoms in it.
- There are a total of 118 elements that have been discovered so far.
- The most prevalent element in the earth crust is OXYgen (46.6 percent), a non-metal.
Compounds
- It’s also a type of matter that may be created by mixing two or more elements in a certain mass ratio.
- Water (H2O) is made up of hydrogen and oxygen in the ratio 1: 8 by mass and may be broken into its constituent parts using proper chemical procedures.
There are two sorts of compounds:
**Inorganic substances **
- These molecules were previously thought to be formed from non-living sources such as rocks and minerals.
- But, with the exception of carbon hydrides (hydrocarbons) and their derivatives, these are the compounds of all elements.Organic compounds
- These molecules, according to previous experts, are obtained from living sources such as plants and animals, or they are buried beneath the soil (e.g., petroleum).
- These are carbon hydrides and their derivatives, according to contemporary definitions.
Mixtures
- These are composed of two or more pure materials. They can have a variety of compositions and can be broken down into their constituents using physical means.
- Mixtures can be homogeneous (when the mixture is consistent throughout) or heterogeneous (when the composition varies) (when composition is not uniform throughout).
The following are some of the most common ways for separating mixtures:
- Filtration is the separation of suspended particles from liquids by putting the mixture into a filter funnel. The solid particles are kept on the filter when the liquid flows through it.
- The process of distillation entails heating a liquid to produce vapours, then cooling the vapours to recover the liquid. This is a method for separating the components of a mixture including volatile chemicals.
- On heating, this is the process of converting a solid into vapours. Sublimate substances, such as iodine, naphthalene, and camphor, have this characteristic. This technique is used to distinguish between sublimate and non-sublimate chemicals.
- The formation of crystals It is the separation of solids with various solubilities in a given solvent.
- Separation magnetic Till’s method relies on the fact that a magnet attracts magnetic components in a combination of magnetic and non-magnetic materials. The non-magnetic material is unaffected by the magnetic field. As a result, it may be utilised to distinguish between magnetic and non-magnetic components.
- Thermolysis The Tills technique is based on gas diffusion rates and is used to separate gases from a gaseous mixture.
Molecules and Atoms
- The atom is the smallest particle of an element capable of participating in a chemical process. It’s possible that it won’t be able to survive on its own.
- The simplest piece of matter with autonomous existence is the molecule.
- It can be homoatomic, such as H2, CI2, N2 (diatomic), or O3 (triatomic), or heteroatomic, such as HCI, NH3, CH3, and so on.
THE MEASUREMENT OF PHYSICAL QUANTITIES
Units
- Two factors are considered when expressing the measurement of any physical quantity:
(i) Its numerical value; (ii) It’s unit.
- A physical quantity’s magnitude is equal to its numerical value multiplied by its unit.
- There are two sorts of units:
Basic units and Derived units
- The basic or fundamental units are the metre, kilogramme,
- Derived units are developed from fundamental units, for example, the density unit is derived from mass and volume units.
Figures of Interest
- The meaningful digits in a measured or computed number are known as significant figures.
- It contains all of the digits that are known with certainty, as well as one that is unknown or estimated.
- The lower the uncertainty, the more meaningful numbers there are in a measurement.
The following are the rules for calculating the number of significant figures:
-
All digits, excluding zeros at the start of a number, are significant.
-
Significant are the zeros to the right of the decimal point. 0.132, 0.0132, and 15.0, for example, are all three significant values.
-
There are an endless number of significant figures in exact integers.
Numerical Results Rounding Off
- The number of significant figures in a number is reduced when it is rounded off.
- Only if the next digit is a 5 is the final digit preserved by 1 and is left as is. 12.696 may be written as 12.7 18.35 can be written as 18.4 13.93 can be written as 13.9 if the following digit is a 4, for example.
Chemical Combination Laws
The following six basic rules control the combining of components to generate compounds:
1. The law of mass conservation (Lavoisier, 1774)
This law asserts that the entire mass of products equals the total mass of reactants throughout any physical or chemical transformation. It is not applicable to nuclear reactions.
2. The definite proportions law (Proust, 1799)
A chemical product acquired from several sources must always include the same percentage of each constituent element, according to this rule.
3. Multiple proportions law (1803; Dalton)
This is how the law works. If two elements can combine to generate more than one compound, they are said to be polymerized. The masses of one element that combine with a fixed mass of the other are in the ratio of tiny whole numbers, for example, in NH3 and N2H4, the fixed quantity of nitrogen requires hydrogen in the ratio 3: 2.
4. The reciprocal proportions law (Richter, 1792)
The ratio in which two elements (say A and 13) mix independently with the same weight of a third element (say C) is the same or simple multiple of the ratio in which they (A and H) combine with each other, according to this law.
When the same compound is made from distinct isotopes of the same element, the laws of definite proportions, multiple proportions, and reciprocal proportions do not apply. H2O and D2O, for example.
5. The law of gaseous volumes of Gay Lussac
It asserts that whenever gases react together under comparable temperature and pressure circumstances. The quantities of reacting gases and products (if gases) have a straightforward whole number relationship.
6. Avogadro’s theorem
It says that under the same temperature and pressure, identical volumes of all gases contain the same number of molecules.
The Atomic Theory of Dalton (1803)
This notion was founded on chemical combination rules.
-
All substances are made up of small, indivisible components called atoms, according to its basic postulates.
-
The atoms in each element are identical and have the same mass. The mass of atoms in various elements varies.
-
During any physical or chemical change, atoms cannot be formed or destroyed.
-
Atoms are combined in some basic numerical ratio to form compounds or molecules.
The Mole Idea
- Ostwald coined the term mole (from the Latin word mole, which means heap).
- A mole is defined as the amount of stuff that includes the same number of constituent particles (atoms, molecules, or ions) as 12 g of carbon dioxide (C-12).
Atomic Mass
- It is an atom’s average relative atomic mass. It expresses how much heavier an atom of that element is as compared to one-twelfth of the mass of a carbon-12 atom.
- Average atomic mass = average atomic mass / 1/12 * mass of a C12 atom
Molecular Weight
- It is a molecule’s mass, or the number of times a molecule is heavier than 1/12th of the mass of a C-12 atom.
- The algebraic total of the atomic masses of all the atoms of various elements present in one molecule may be used to compute a substance’s molecular mass, which is an additive feature.
Stoichiometry
- Stoichiometry (from the Greek phrase ‘to measure an element’) refers to the relative quantities in which reactants react and products are created.
Yield Percentage
- Because of the presence of certain side reactions, the actual yield of a product in any reaction is frequently lower than the theoretical yield.
- Actual yield / projected yield * 100 = percent yield
Formulae based on empirical data and Molecular Formulae
- The simplest formula of a chemical is the one that gives the simplest whole number ratio of atoms present in one molecule, for example, CH is the empirical formula of benzene (C6H6).
- The molecular formula of benzene is C6H6. It is the exact formula of a compound that shows the total number of atoms of component elements.