CBSE Class 11 Chemistry Chapter 11 Revision Notes
Chapter 11: P – block Elements Revision Notes
Elements of the p-Block
- P-block elements are elements that belong to groups 13 through 18 of the periodic table.
- ns2 np1-6 is the general electrical configuration (except for He)
Elements of Group 13: Boron’s Family
- ns2np1 is the outer electronic configuration.
Atomic and Ionic Radii:
- Group 13 elements have lower atomic and ionic radii than their alkali and alkaline earth metal counterparts.
- The effective nuclear charge grows as you move from left to right in a period, and the outside electrons are drawn more forcefully towards the nucleus. As a result, the atomic size shrinks.
- With each successive element, the atomic and ionic radii are projected to rise due to the addition of a new electron shell.
- Due to the existence of poor shedding 10d-electrons in gallium, its atomic radius is less than that of Al.
Ionisation enthalpies:
- In the same time, the first ionisation enthalpies of the elements in group-13 are lower than those of the elements in group-2.
- Because removing the p-electron is significantly simpler than removing the s-electron, the initial ionisation enthalpies I H1) of the elements of group 13 are lower than those of group 2.
- The first-ionization enthalpies I H1) fall as one moves down group 13 from B to Al due to an increase in atomic size and screening effect, which exceed the impact of increasing nuclear charge.
- Because of the capacity of d- and f-electrons, which have a low screening effect, to compensate for the rise in nuclear charge, there will be a discontinuity in the ionisation enthalpy values between Al and Ga and between In and Tl.
- Electronegativity declines from B to Al and then rises as you move down the group.
- This is due to differences in the size of the elements’ atoms.
Physical Characteristics
(i) Boron has a high melting point due to its strong crystalline structure. The rest of this family's members have a low melting point.(ii) Boron is a non-metallic solid that is exceedingly hard and black in colour.(iii) Soft metals with a low melting point and excellent electrical conductivity are also members of this family.Chemical Characteristics
- The first two elements, boron and aluminium, have only a +3 oxidation state in their compounds, whereas the remaining elements in this group, gallium, indium, and thalium, have both a +1 and a +3 oxidation state, indicating that they have various oxidation states.
- The stability of the +3 oxidation state decreases as we move down the group, while the stability of the +1 oxidation state gradually increases.
- Carbon (C), silicon (Si), Germanium (Ge), tin (Sn), and lead (Pb) are all members of Group 14 of the Carbon Family (Pb).
- The ns2np1 electrical arrangement is the most common in the carbon family.
Carbon is the seventeenth most prevalent element in the earth’s crust by weight.
(i) It comes in three forms: coal, graphite, and diamond. It can be found in metal carbonates, hydrocarbons, and carbon dioxide gas (0.03 percent) in the atmosphere in its combined state.
(ii) The stable iosotopes 12C and 13C, as well as the third isotope 14C, are found in naturally occurring carbon. Radiocarbon dating uses 14C, a radioactive isotope having a half-life of 5770 years.
- From C to Si, the covalent radius is projected to grow. There is a modest rise from Si to Pb.
- The addition of a new energy shell in each consecutive element is the reason behind this. Due to inadequate shielding of the valence electrons by the intervening d- and f orbitals, the increase in covalent radii from Si to Pb is minimal.
Ionization Enthalpy
Group 14 elements have greater initial ionisation enthalpies than their comparable group 13 elements.
Because the effective nuclear charge increases, the size of the atoms shrinks. As you move down the group from carbon to tin, the first ionisation enthalpy lowers.
The drop is abrupt from carbon to silicon, whereas the initial ionisation enthalpy of lead is somewhat higher than that of tin.
Electronegativity
- Because the elements in Group 14 are smaller than those in Group 13, they are somewhat more electronegative than those in Group 13.
- It’s roughly the same from Si to Pb.
- The impact of increased nuclear charge surpasses the shielding effect owing to the existence of more 4f- and 5d-electrons, resulting in a little increase in ionisation enthalpy from Sn to Pb.
Physical characteristics:
(i) Group 14 elements consist entirely of solids. They have a lower metallic content than group 13.(ii) Group 14 elements have high melting and boiling points.Chemical characteristics:
- The oxidation state of carbon and silicon is usually +4. In the +4 state, germanium creates stable compounds, but in the +2 state, it forms just a few compounds.
- In both oxidation states, tin produces compounds. Lead compounds in the +2 state are stable, but those in the +4 state are powerful oxidizers.
Anomalous Behaviour of Carbon
Carbon is distinct from the other members of its family. The major cause of the strange behaviour is:
(i) atomic and ionic sizes that are very tiny(ii) a greater enthalpy of ionisation(iii) In the valence shell, there are no d-orbitals.(iv) An increase in electronagativity.CARBONS ’ ALLOTROPES
- Allotropy refers to an element’s ability to exist in two or more forms with differing physical attributes but similar chemical properties. Different forms are referred to as allotropes.
There are two allotropic forms of carbon:
(i) Crystalline (ii) Amorphous
Diamond, Graphite, and Fullerenes are crystalline forms of carbon.
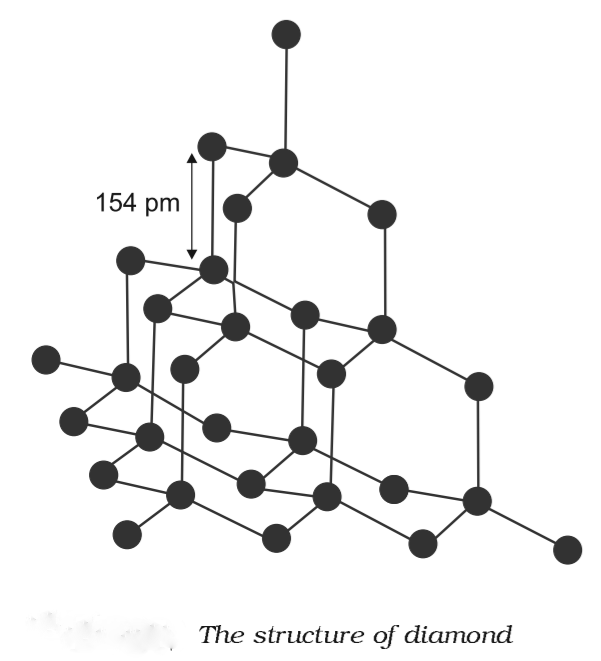
Diamond
- Each carbon atom in a diamond undergoes sp3 hybridisation. Each carbon atom is connected to four other carbon atoms in a tetrahedral arrangement.
- The length of the C—C bond is 154 pm.
Properties:
(i) It is the world's hardest material.(ii) It is utilised as an abrasive for sharpening hard equipment in the dyeing and tungsten filament manufacturing industries.Graphite
- It is a sp2-hybridized carbon material.
- The two-dimensional sheet-like structure of graphite is made up of a series of hexagonal rings bonded together.
- The van der Waals forces hold the layers together, and the gap between them is 340 pm.
Properties:
(i) The sheet of graphite conducts electricity.(ii) It is extremely soft and slick.(iii) Used as a dry lubricant in machines that operate at high temperatures and cannot be lubricated with oil.Fullerenes
- Three scientists, E. Smalley, R.F. Curl, and H.W. Kroto, discovered fullerenes together.
Preparation:
- Fullerenes are made by heating graphite in the presence of an inert gas such as helium or argon in an electric arc.
- The sooty substance generated by the condensation of vapourised Cn tiny molecules is mostly composed of C70 with traces of other fullerenes with even numbers of carbon atoms up to 350 or higher.
- Fullerenes are a kind of molecule that resembles a cage. Buckminsterfullerenes are C60 molecules that have the form of a soccer ball. It’s the most dependable.
- It has 20 rings with six members and 12 rings with five members.
- Six-membered rings are fused to each other as well as to five-membered rings, whereas five-membered rings are only attached to six-membered rings.
- The carbon atoms are of the same size and undergo sp2-Hybridization.
Properties:
(i) Fullerenes are soluble in organic solvents because they are covalent.(ii) Platinum complexes are also formed.Carbon
- Coke in amorphous allotropic forms is a greyish black hard solid generated during destructive distillation.
- Wood charcoal is made by burning wood with a high heat and a little amount of oxygen.
- Animal charcoal is made by distilling bones in a damaging manner.
Uses of carbon:
(i) Graphite fibre is utilised to make high-quality sporting equipment such as tennis and badminton rackets, as well as fishing rods.(ii) Graphite is used to make electrodes for batteries and industrial electrolysis because it is an excellent conductor.(iii) Because of its porous nature, activated charcoal is utilised in gas masks to absorb hazardous gases. It's used to remove the colour from sugar.(iv) Carbon black is used as a filler in automibile tyres and as a black pigment in black ink.(v) In metallurgy, coke is often utilised as a reducing agent.(vi) A diamond is a valuable gemstone.