CBSE Class 11 Chemistry Chapter 13 Revision Notes
Chapter 13: Hydrocarbons Revision Notes
- Hydrocarbon: The term “hydrocarbon” refers to a carbon-hydrogen compound.
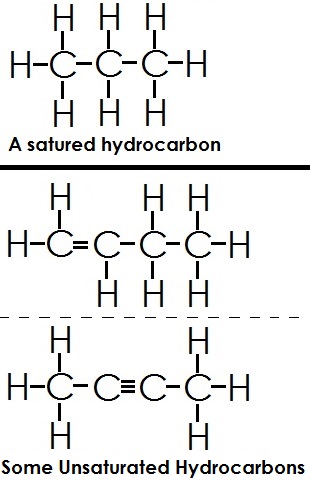
- Saturated Hydrocarbon: If a hydrogen contains only C—C single bonds, it is said to be saturated. E.g. Ethane CH3—CH3
- Unsaturated Hydrocarbon: If a hydrogen contains double (C=C) or triple bonds between the Carbon atoms they are said to be unsaturated hydrocarbons.
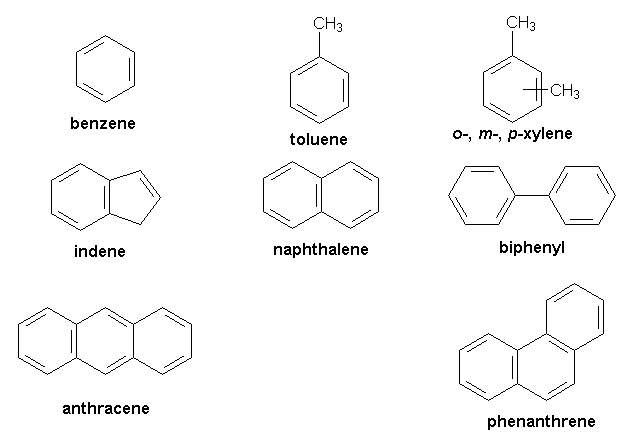
- Aromatic Hydrocarbon: Aromatic compounds include benzene and its derivatives.
- Alicyclic Compounds: Alicyclic or carbocyclic compounds are cyclic compounds that contain only carbon atoms.
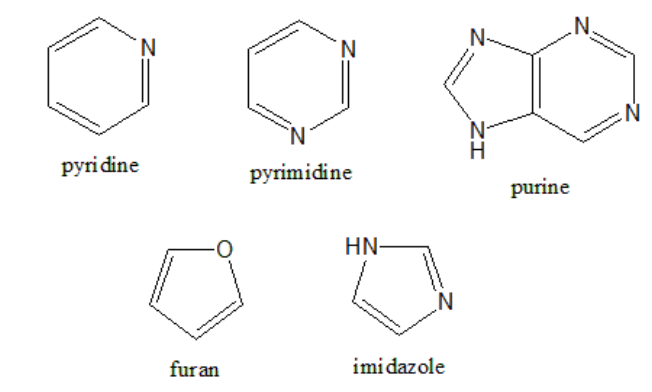
- Heterocyclic Compounds: Heterocyclic compounds are cyclic compounds in which the ring atoms are carbon and another element (for example, N, S, or O).
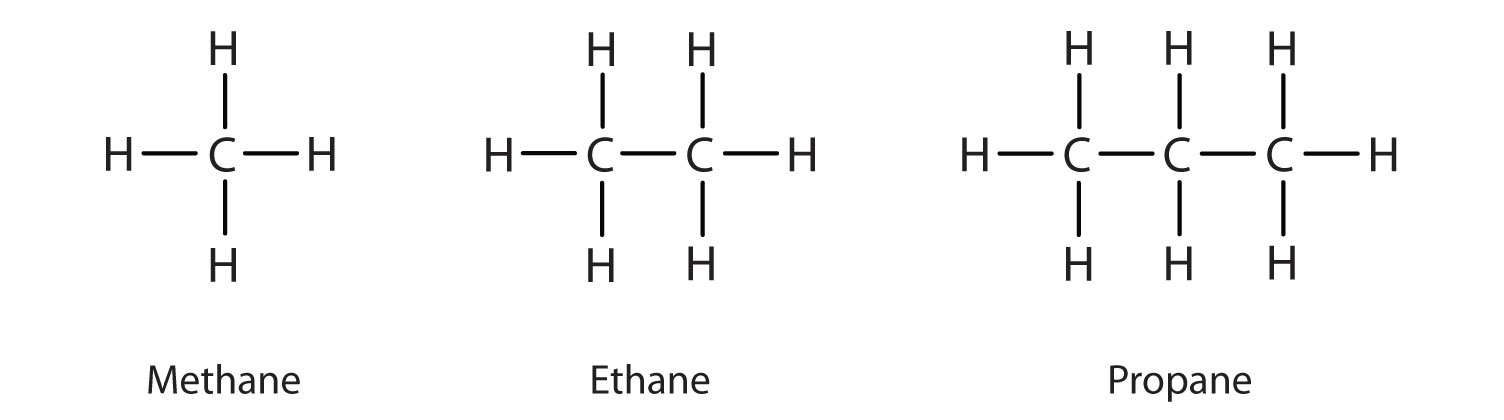
- Alkanes: Alkanes are the most basic organic compounds, consisting solely of carbon and hydrogen.
- CnHC2n+2 (where n = 1, 2, 3, etc.) is the general formula for them.
- Single covalent bonds bind the carbon atoms in their molecules to one another. Alkanes are also known as saturated hydrocarbons because their carbon skeleton is fully saturated with hydrogens.
- Alkanes have a lot of C—C and C—H bonds. As a result, this group of hydrocarbons is chemically inert. As a result, they’re sometimes referred to as paraffins. Hydrocarbons make up the first three members of this class.
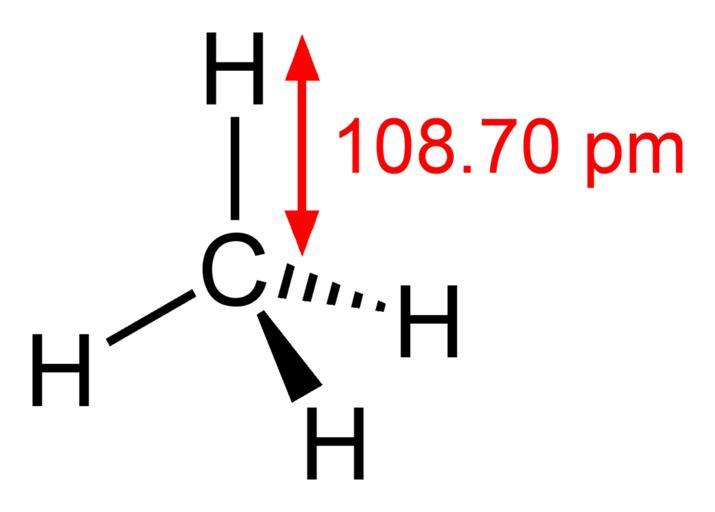
- Carbon forms single bonds with four hydrogen atoms in methane. The H—C—H bond angles are all 109.5 degrees. The structure of methane is tetrahedral. Head-on overlapping of sp3 hybrid orbitals of carbon and Is orbitals of hydrogen atoms forms C—C and C—H bonds.
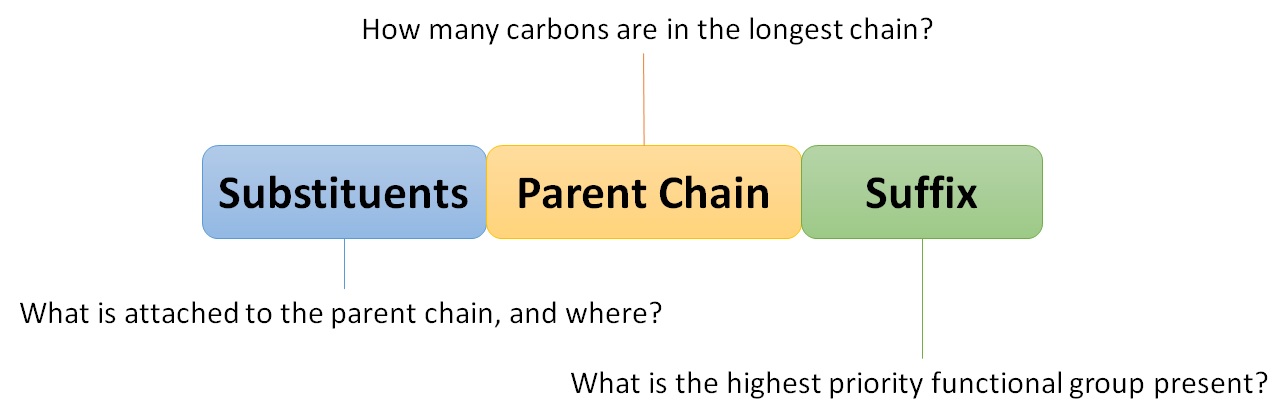
Nomenclature Guidelines
- To write the IUPAC names from the structural formulas, use the step-by-step procedure below. Take a look at the following formula for a structural formula:
Step 1: Determine which chain is the longest.
Step 2: Count the links in the chain: From left to right, the chain is numbered. The attached alkyl group receives the lowest numbers as a result of this.
Step 3: Determine the alkyl group.
- Always keep in mind that
(a) commas are used to separate numbers.
(b) Hyphens are used to separate numbers from names;
(c) The prefixes di and tri are ignored when alphabetizing substituent names.
Relative Stability of Conformations
- There are maximum repulsive forces, minimum energy, and maximum molecule stability in the staggered form of ethane.
- When the staggered form becomes eclipsed, the electron clouds of the carbon hydrogen bonds become closer to each other, increasing electron cloud repulsions. As a result, the molecule requires more energy and thus has lower stability.
- The magnitude of torsional strain is determined by the angle of rotation about the C—C bond. This angle is also known as torsional angle or dihedral angle.