CBSE Class 11 Chemistry Chapter 3 Revision Notes
Chapter 3: Classification of Elements and Periodicity in Properties Revision Notes
**Dobereiner’s Triads **
- Dobereiner ordered several elements with comparable characteristics in groups of three in 1829, with the middle element’s atomic mass almost equal to the average atomic masses of the first and third elements.
Dobereiner’s Trids’ Limitations
- Dobereiner’s triads were useful for grouping together components with comparable qualities, but he couldn’t put all of the elements known at the time into triads.
Newlands’ Law of Octaves
- When elements are ordered in order of increasing atomic masses, John Newlands suggested the law of octaves, which states that every eighth element has characteristics comparable to the first.
- Newlands coined the term “rule of octaves” to describe the connection between musical notes.
Newlands’ Law of Octaves has certain limitations.
(i) Only up to the element calcium was this categorization effective. Following that, every eighth element did not have the same attributes as the member in the group above it.
(ii) When noble gas elements were found later, their inclusion in the table threw the entire system into disarray.
MENDELEEVS PERIODIC TABLE
- Mendeleev’s Periodic Law states that the physical and chemical characteristics of elements follow a regular pattern.
- Their atomic masses are a function of their mass.
- Mendeleev grouped the known elements in order of increasing atomic masses at the time.
- The periodic table was named after this layout.
- In vertical rows named groups, elements with comparable features were found. The vertical
- Periods were used to refer to rows.
(i) The elements in the periodic table are organised into vertical rows called groups and horizontal rows called periods.
(ii) The Roman numerals I, II, III, IV, V, VI, VII, VIII, and zero designate nine groups. Group VIII is made up of nine components organised into three triads. The zero group comprises elements that are either inert or noble gases, and all of the elements in the group have zero valency.
(iii) In Mendeleev’s periodic chart, there are seven periods (numbered 1–7) or horizontal rows.
Importance of Mendeleev’s Periodic Table
(i) This made element study more systematic in the sense that if the qualities of one element in a group are known, the properties of others can be predicted.
(ii) This aided in the eventual discovery of these elements to a large extent.
(iii) Mendeleev used anticipated locations and attributes to adjust the atomic weights of some elements.
Defects in Mendeleev’s Periodic Table
(i) Hydrogen, like alkali metals, is classified as a group IA element. However, it has several features with group VII A halogens. As a result, its place in Mendeleev’s periodic table remains debatable.
(ii) Although the elements in Mendeleev’s periodic table are ordered by atomic mass, in certain circumstances the element with the higher atomic mass comes before the element with the lower atomic mass.
(iii) We already know that an element’s isotopes have distinct atomic weights but the same atomic number. Because the periodic table was created based on the rising atomic masses of the elements, all of the isotopes of a given element must have been assigned to distinct locations.
(iv) According to Mendeleev, elements in the same group must have characteristics that are similar. However, there is no resemblance between the components in two sub-groups of the same group.
Modern Periodic Law
- The periodic function of the atomic numbers determines the physical and chemical characteristics of the elements.
 Source:
Source:
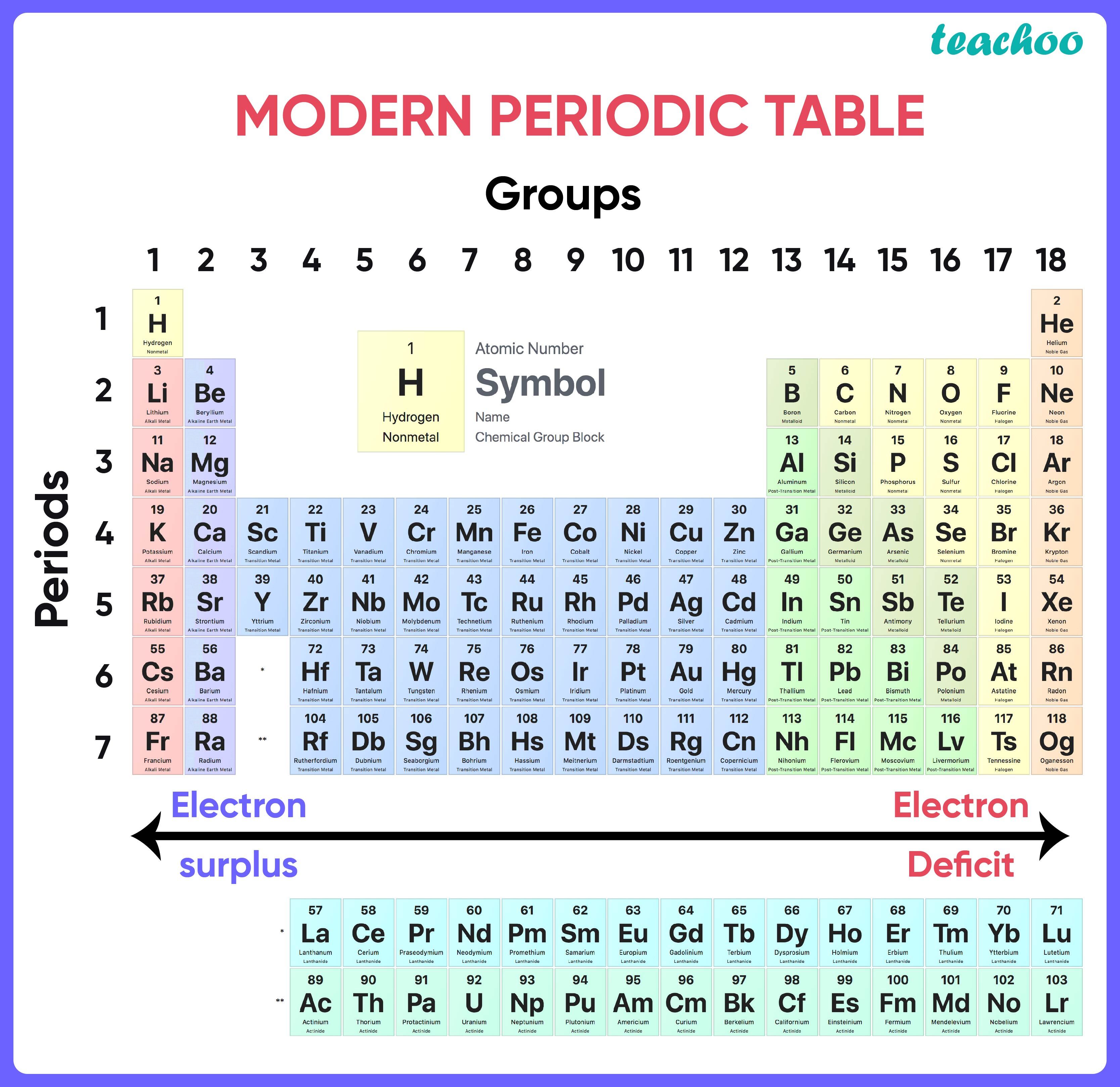
**The Periodic Table in Its Current Form **
- Modern periodic law is used to create the long version of the periodic table, commonly known as the Modem Periodic Table.
- The elements have been organised in this table in order of increasing atomic numbers.
ELECTRONIC ARRANGEMENTS OF THE ELEMENTS IN THE s, p, d, and f blocks
s-block
- In these elements, the last electron reaches the s-orbitals.
- A general outer shell electrical configuration of s-block components is ns1-2, where n = 2-7.
- They are softer metals with low melting and boiling points.
- They have low ionisation enthalpies and are electro positive (energies).
- They rapidly lose their valence (outermost) electrons, resulting in the formation of +1 (alkali metals) and +2 ions (in case of alkaline earth metals).
- Metals with a high degree of reactivity. The metallic nature and responsiveness become more obvious as we proceed through the group. Due to their extreme reactivity, they are seldom found pure in nature.
- All s-block element compounds are ionic, with the exception of beryllium.
p-block
- ns2np1-6 is a general electrical arrangement.
- The compounds of the d-block elements are predominantly covalent in character.
- They come in a variety of oxidation states.
- As the period proceeds from left to right, the components’ non-metallic nature increases.
- Within a group, the responsiveness of components tends to diminish.
- At the conclusion of each cycle, a noble gas element with a closed valence shell ns2np6 configuration is present.
- The metallic element gets increasingly obvious as we progress through the group.
d-block
- (n-1)d1-10ns1-2 is a common electrical arrangement.
- They’re all metals with high melting and boiling points.
- The compounds of d-block elements are frequently paramagnetic in nature.
- They mostly create coloured ions with a wide range of valences (oxidation states).
- The d-block elements are known as transition elements because they have incompletely filled d-orbitals in their ground state or any of the oxidation states.
- Catalysts are usually made up of d-block element compounds.
f-block
- n-2f1-14((n2)f(014)(n1)d(01)ns2 is the general electrical arrangement.
- Because electrons in d-block transition elements are filled in (n – 1) d subshells, but electrons in f-block inner transition elements are filled in (n – 2) f subshells, which is one inner subshell, they are termed inner transition elements.
- Two rows of elements at the bottom of the Periodic Table are the Lanthanoids Ce (Z = 58) – Lu (Z = 71) and the Actinoids Th (Z = 90) – Lr (Z = 103).
- All of them are composed of metal. The components in each series have a lot of the same qualities.
- A considerable number of radioactive elements are found in the actinoid series.
Periodic Trends in Properties of Elements
Physical Property Changes
Atomic Radii: The distance between the nucleus’s centre and the outermost shell containing the electrons is measured in atomic radii. Three distinct types of atomic radii are employed depending on whether an element is a non-metal or a metal. These are the following:
(a) Covalent Radius: Half the distance between the nuclei of two atoms held together by a purely covalent single bond.
(b) Ionic Radius: This is the effective distance from an ion’s nucleus to which it has an influence on the ionic bond.
(c) Van der Waals Radius: The weak van der Waals forces of attraction hold Noble gas atoms together. The van der Waals radius is equal to half the distance between the nuclei of noble gas atoms.
(d) Metallic Radius: In the metallic lattice, it is defined as half of the internuclear distance between two neighboring metal ions.
Ionic Radius
- The distances between cations and anions in ionic crystals can be used to estimate the ionic radii.
- In general, element ionic radii follow the same pattern as atomic radii.
- The production of a cation occurs when an electron is removed from an atom. The cation’s radius is always lower than the atom’s.
- An anion is formed when an electron is gained. The anion’s radius is always greater than the atom’s radius.
- Isoelectronic Species: We name isoelectronic species atoms and ions that have the same number of electrons. O2-, F–, Na+, and Mg2+, for example, all have the same amount of electrons (10). Because of their various nuclear charges, their radii would be varied.
Electronegativity
- Electronegativity is a qualitative measure of an atom’s capacity to attract shared electrons to itself in a chemical combination.
- It is not a quantifiable quantity, unlike ionisation and electron gain enthalpy.
- However, a variety of numerical scales for electronegativity of elements have been created, including the Pauling scale, Milliken-Jaffe scale, and Allred Kochow scale.
- Any particular element’s electronegativity is not constant; it fluctuates based on the element to which it is linked.
Periodic Trends in Chemical Properties along a Period
(i) Characteristics of metal: Decrease over a period maxima on the extreme left (alkali metals).
(ii) Non-metallic character: Becomes more non-metallic over time. (From the left to the right)
(iii) Basic nature of oxides: Decreases in a period from left to right.
(iv) Acidic character of oxides: Increases with time from left to right.
Differences in Moving Down a Group from Top to Bottom
(i) It has a metallic feel about it. From top to bottom, the ionizatiort energy of the elements in a group decreases due to an increase in atomic size and hence a drop in the ionizatiort energy.
(ii) Characteristics that aren’t metallic. Reduces the size of a group in general. From top to bottom in a group, the electronegativity of elements diminishes.
(iii) The fundamental character of oxides. The fundamental nature of oxidise naturally rises as the metallic character or electropositivity of elements increases from top to bottom in a group.
(iv) The acidic nature of oxides. As the non-metallic property of elements drops from top to bottom in a group, it generally decreases.
(v) Metals’ reactivity. In general, the number of people in a group grows. Because the potential to lose electrons is increasing.
(vi) Non-metal reactivity. The higher the electro-negativity of non-metals, the greater their reactivity. The reactivity of non-metals in a group reduces as their electronegativity falls from top to bottom.
]]>