CBSE Class 12 Chemistry Chapter 9 Revision Notes
Chapter 9: Coordination Compounds Revision Notes
- Coordination compounds have a central atom (or cation) that is coordinated to a number of anions or neutral molecules, and they usually retain their identity in both solution and solid state.
- These can be positively, negatively, or neutrally charged species, such as [Co(NH3)6]3+, [NiCl4]2-, [Ni(CO)4], and so on.
- In 1893, Werner proposed a theory to explain coordination compound structure and bonding.
- According to this theory metals have two types of valencies in coordination compounds: primary valency and secondary valency.
- Primary valencies are ionisable.
- Secondary valencies cannot be ionized.
- This theory was only partially successful, and it failed to explain many aspects of coordination compounds.
- Today, such spatial arrangements are known as coordination polyhedra.
- The ions outside the square brackets are called counter ions, and the species within the square brackets are coordination entities or complexes.
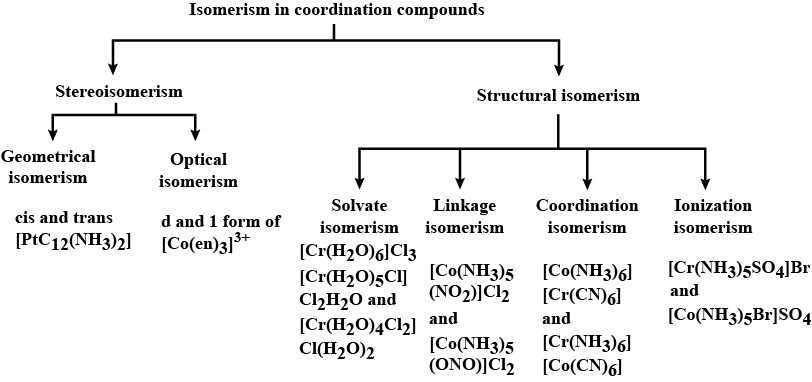
- Isomers are compounds that share the same molecular formula but differ in their structural arrangements.
Types of Isomerism
Magnetic characteristics
- Inner orbital (low spin) complexes are those in which (n-1) d, ns, and np-orbitals are hybridized to form metal hybrid orbitals, such as [Fe(CN)6]4+, [CO(NH3)6]3+, [Cr(NH3)6]3+, [Fe(CN)6]2+, [Fe(H20)6]2+, [(MnCCN)6]3
- Outer orbital (high spin) complexes, such as [MnF6]3-, [FeF6]3-, [Ni(NH3)6]2+, [Ni(H20)6]2+, etc., are complexes in which the metal’s hybrid orbitals are formed by hybridization of ns, np, and nd-vacant orbitals.
Assumptions of Crystal Field Theory
- Point charges are assumed for the ligands.
- The interaction between the point charges and the central metal’s electrons is electrostatic.
- In an isolated gaseous metal ion, the 5d-orbitals have the same energy, indicating that they are degenerate.
- A coordination compound’s [MLn] stability is measured in terms of its stability constant.
Drawbacks of Crystal Field Splitting
- Because ligands are assumed to be point charges, anionic ligands should have the greatest splitting effect.
- But anionic ligands are found at the bottom of the spectrochemical series.
- The covalent nature of the bonding between the ligand and the central atom is not taken into account.
- Metal carbonyl bonding: The metal carbon (M – C) bond has both the – and -bond character in metal carbonyl.
Importance of Coordination Compounds and their Applications
- Used in a variety of quantitative and qualitative chemical tests.
- In the extraction of metals such as silver and gold.
- The formation and subsequent decomposition of metal coordination compounds can be used to purify metals such as Ni.
- Chlorophyll, a magnesium coordination compound, is the pigment responsible for photosynthesis in biological systems.
- Haemoglobin is a ferrous coordination compound that serves as an oxygen carrier.
- In medicinal chemistry, an example of chelate therapy.