CBSE Class 12 Physics Chapter 11 Revision Notes
Chapter 11: Dual Nature of Radiation and Matter Revision Notes
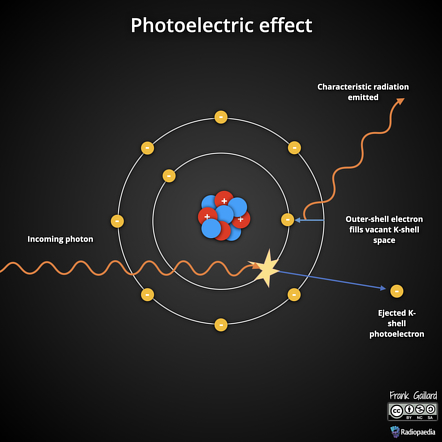
- Photoelectric effect: The photoelectric effect is the phenomenon of photoelectron emission from a metal surface when a light beam of correct frequency is incident on it.
- Photoelectrons are the emitted electrons, and photoelectric current is the current produced as a result.
- Heinrich Hertz discovered photoelectric emission in 1887 while conducting an electromagnetic wave experiment. When the emitter plate was lit up by ultraviolet light from an arc lamp, his experiment on the production of electromagnetic waves by means of spark across the detector loop was enhanced.
- Lenard observed that when ultraviolet radiation is allowed to fall on the emitter plate of an evacuated glass tube enclosing two electrodes, current flows. The current flows ceased as soon as the ultraviolet radiations were turned off. These findings suggest that when ultraviolet radiation strikes the emitter plate, electrons are ejected, and the electric field attracts them to the positive plate.
Photoelectric Effects Terminology
-
Free Electrons: The electrons in the outer shells (valence electrons) of metals are loosely bound to the atoms, allowing them to move freely within the metal surface but not beyond it. Free electrons are these types of electrons.
-
Electron Emission: The phenomenon of electron emission from a metal’s surface is known as electron emission.
-
Photoelectric Emission: It is the emission of electrons from the surface of a metal when suitable frequency light radiation falls on it.
-
Work Function: The work function of a metal is defined as the minimum amount of energy required to simply eject an electron from the metal’s outermost surface.
-
Cut-off Potential: The minimum negative (retarding) potential V0 given to the plate for which the photoelectric current becomes zero for a particular frequency of incident radiation is known as the cut-off or stopping potential.
-
Cut-off Frequency: is the frequency at which a signal is cut off. The threshold frequency or cut-off frequency of a material is the lowest frequency of light that can emit photoelectrons from that material.
-
Cut-off Wavelength: The threshold wavelength or cut-off wavelength of a material is the maximum wavelength of light that can emit photoelectrons from that material.
-
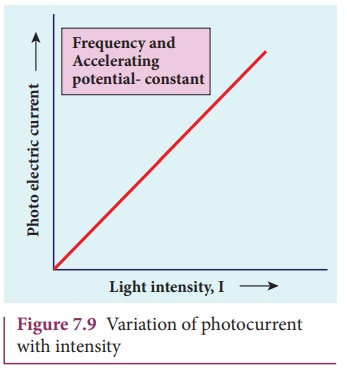
The Effect of Intensity of light on Photocurrent: The photoelectric current increases linearly with increasing incident light intensity for a fixed frequency of incident radiation.
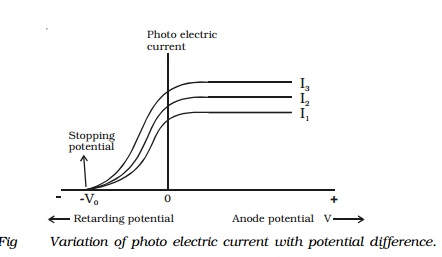
- Effect of Potential on Photoelectric Current: The photoelectric current increases as the potential applied to the collector increases for a fixed frequency and intensity of incident light. When all of the photoelectrons reach plate A, the current reaches its maximum value, which is referred to as saturation current.
- The Effect of Frequency of Incident Radiation on Stopping Potential: We collect radiations of varying frequencies but equal intensity. We examine the variation of photoelectric current against the potential difference between the plates for each radiation.
Photoelectric Emission Laws
- For a given material and incident radiation frequency, the photoelectric current is proportional to the incident light intensity in terms of photoelectrons ejected per second.
- For a given material and incident radiation frequency, saturation current is found to be proportional to incident radiation intensity, whereas stopping potential is independent of incident radiation intensity.
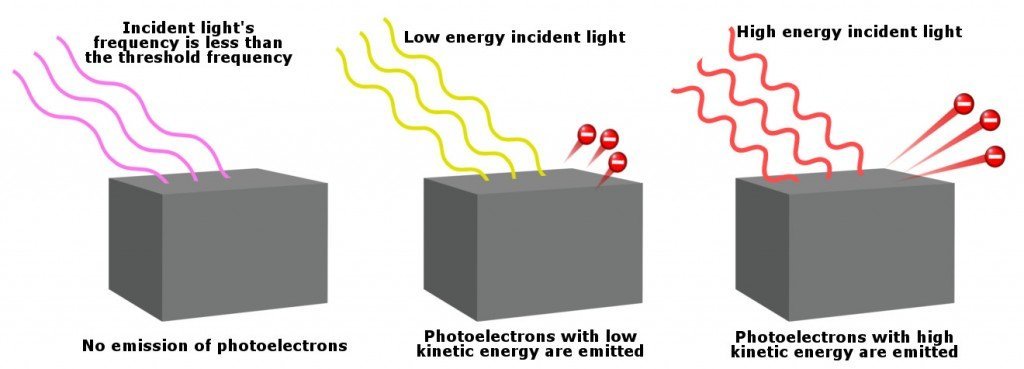
- There is a certain minimum frequency of incident radiation for a given material below which no photoelectrons are emitted. The frequency at which this occurs is known as the threshold frequency.
- The maximum kinetic energy of the photoelectrons emitted or equivalent stopping potential is independent of incident light intensity above the threshold frequency and is dependent only on the frequency (or wavelength) of the incident light.
- Photoelectric emission occurs in a split second. The time lag between the incidence of radiation and photoelectron emission is extremely short, less than 10-9 s.