CBSE Class 9 Science Chapter 4 Revision Notes
Chapter 4:Structure of Atoms Revision Notes
An atom is made up of Protons, neutrons, and electrons. The mass and charge of the atoms are provided by these fundamental components. The proton and neutron make up the nucleus, with the electron circling around it.
INTRODUCTION TO STRUCTURE OF AN ATOM
Atoms
- Atoms are the fundamental components of matter. The three subatomic particles are Proton, neutron, and electrons which make up the smallest unit of matter.
Daltons Atomic Theory
- The first recorded proof that atoms exist is Dalton’s Atomic Theory.
- Dalton rationalised the many principles of chemical combination using his theory.
- Dalton’s theory was founded on the idea that the weights of various elements’ atoms might be used to differentiate them.
Limitations
- An atom’s indivisibility was disproved since it can be further split into protons, neutrons, and electrons.
- Atoms of the same element are similar in every way, yet isotopes of the same element have differing masses.
- Dalton’s theory was founded on the idea that the weights of various elements’ atoms might be used to differentiate them.
J J Thomson Experiments
- In 1897, he discovered electrons.
- It demonstrated that the atom may be broken down into even smaller pieces.
- His discovery was the first step toward a complete atomic model.
- An atom is a homogeneous sphere with both positive and negative charges (owing to the existence of protons) (due to presence of electrons).
- Because the negative and positive charges are of equal size, the atom as a whole is electrically neutral.
- A negatively charged component of an atom that lives beyond the nucleus is called an electron. When compared to the mass of a neutron or proton, each electron carries one unit of negative charge and has a relatively little mass.
- JJ Thomson demonstrated that the cathode ray responds to both magnetic and electric fields using cathode ray tubes.
- He deduced that the ray was made up of negatively charged particles because it was attracted to a positive electric plate put over the cathode ray tube (beam deflected toward the positive plate).
- These negative particles were dubbed “electrons” by him.
Limitation:
- The model was unable to explain why protons and electrons were positioned so close together in the atom.
Goldstein, Eugene:
- In 1886, E. Goldstein identified new radiations in a gas discharge and named them canal rays. These rays were positively charged radiations, and their discovery led to the discovery of yet another subatomic particle.
- Using a Cathode Ray Tube, he investigated “canal rays,” which possessed electrical and magnetic characteristics that were the polar opposite of an electron.
- Canal Rays: Canal rays are positively charged radiation generated in the discharge tube at low pressure and high voltage.
Protons
- Protons are positively charged subatomic particles found in canal rays (p).
Scattering Experiments by Rutherford:
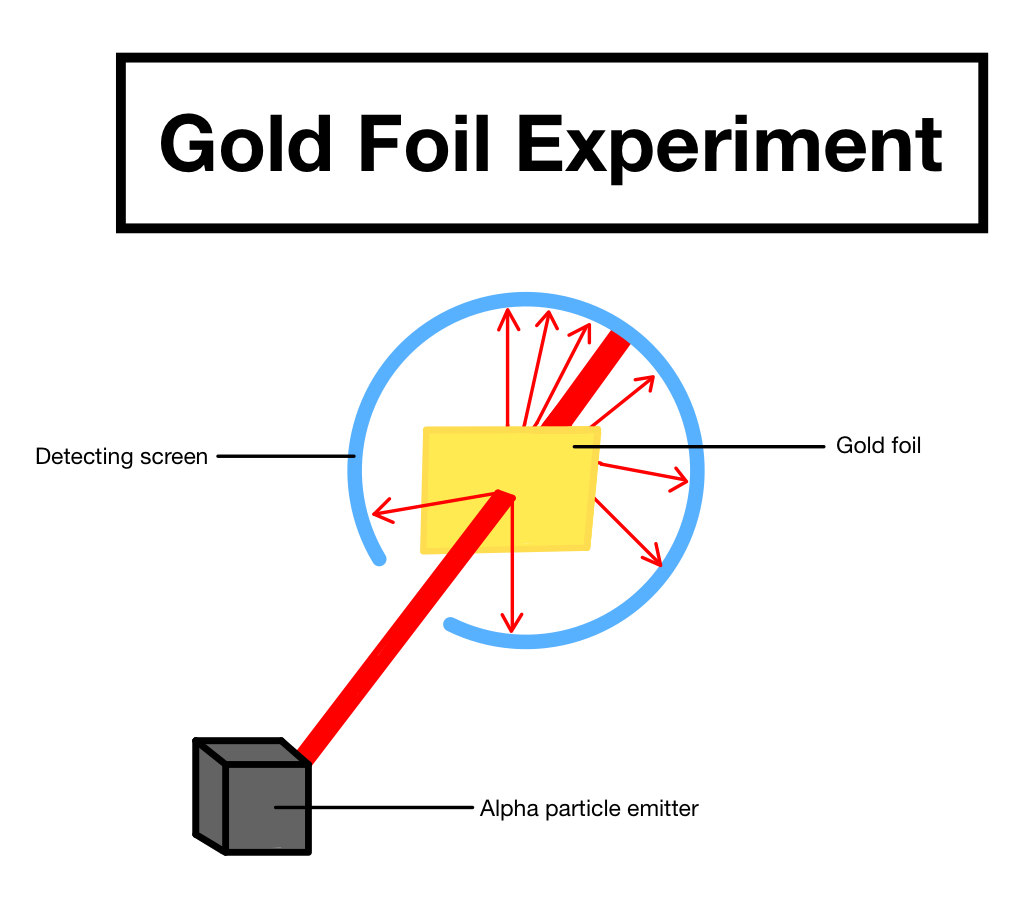
- Rutherford performed an experiment in which he sprayed alpha particles of positively charged Helium onto a thin gold foil.
- His findings were as follows: a large percentage of the -particles blasted towards the gold sheet flowed through it without deflection, and hence the majority of the space in an atom is unoccupied.
- The gold sheet deflected some of the -particles at minor angles, causing the positive charge in each atom to be unevenly distributed.
- In an atom, the positive charge is concentrated in a relatively small volume.
- Only a few -particles were deflected back, implying that only a few -particles had almost 180o deflection angles. As a result, the positively charged particles in an atom occupy a very little volume in comparison to the entire volume of the atom.
Rutherford’s atomic model
- From the -particle scattering experiment, Rutherford came up with the following atom model:
- An atom has a nucleus, which is a positively charged centre. The nucleus contains nearly all of an atom’s mass.
- (ii) The electrons have well-defined orbits around the nucleus.
- (iii) In comparison to the size of the atom, the nucleus is quite tiny.
Limitations of the Rutherford’s model
- He revealed that electrons in an atom move in well-defined orbits around the nucleus. In a circular orbit, particles would feel the acceleration.
- As a result, the spinning electron loses energy and falls into the nucleus.
- However, this is impossible since the atom would be unstable and matter would cease to exist in the form we know it.
The Atomic Model of Niels Bohr
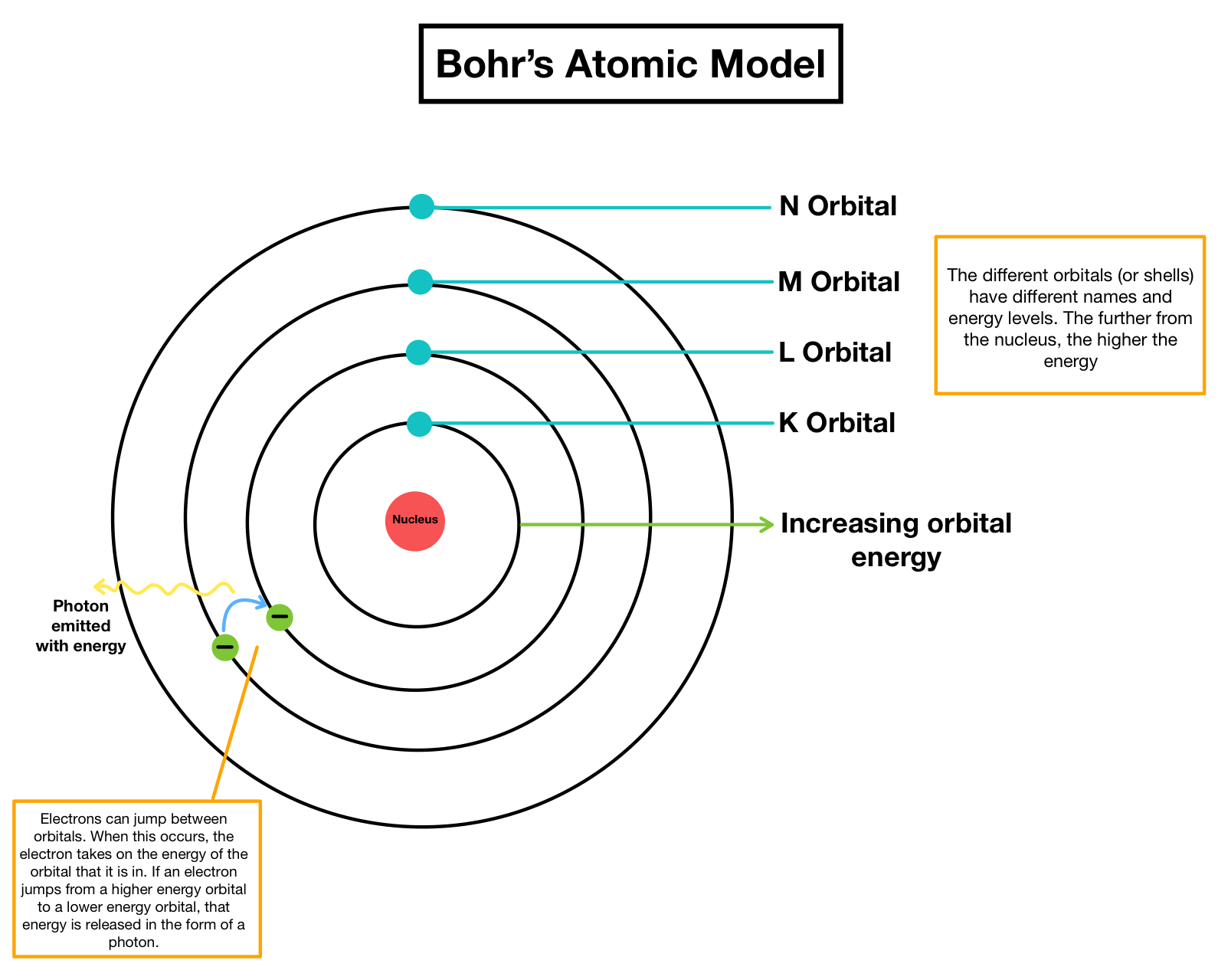
To address the criticisms leveled about Rutherford’s model, Bohr devised the following postulates:
- Electrons circle the nucleus in stable orbits and do not emit radiant energy. Each orbit has a certain energy level, which is referred to as an energy shell or energy level.
- K, L, M, and N shells denote an orbit or energy level. The electron is considered to be in the ground state when it is at its lowest energy level.
- When an electron goes from one orbit or energy level to another, it emits or absorbs energy.
- When it moves from a higher to a lower energy level, it creates energy, and when it leaps from a lower to a higher energy level, it absorbs energy.
ORBITS
- Orbits are energy shells that encircle the nucleus and in which electrons spin.
- The distribution of electrons in various orbits
- Bohr and Bury recommended the distribution.
- The formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,…., gives the maximum number of electrons present in a shell.
- The maximum number of electrons in each orbit is as follows: the first orbit has 212=2, the second orbit has 22Msup>2=8, the third orbit has 232=18, the fourth orbit has 242=32, and so on.
- From lower to higher energy levels, the shells are always filled in a step-by-step manner. Electrons aren’t filled in the following shell until the shells before it have been filled.
VALENCY
- The valence electrons are the electrons that make up an atom’s outermost shell.
- The valency of an atom refers to its capacity for combining or proclivity to combine and form molecules with atoms of the same or different elements.
- Chemical activity is minimal in atoms with a totally filled outermost shell.
- Their valency (or combining capability) is 0.
- For example, we know that the outermost shell of hydrogen has one electron, while the outermost shell of magnesium has two.
- As a result, hydrogen has a valency of 1 since it may readily drop an electron and become stable.
- Magnesium, on the other hand, has a value of 2 because it can readily shed 2 electrons while maintaining stability.
Number of atoms
- The atomic number is the number of protons contained in the nucleus of an atom. The letter ‘Z’ stands for it.
An atom’s mass number and representation
- Because the nucleus contains protons and neutrons, the mass number is the sum of these protons and neutrons.
ISOTOPES AND ISOBARS
- Isotopes are atoms of the same element that have the same atomic number (number of protons) but differ in mass (number of protons+neutrons).
- Isobars are atoms of various elements with distinct atomic numbers that have the same mass number.
